Question: Please use Matlab to solve this question: 56. The ideal gas law provides one way to estimate the pressures and vol- umes of a gas
Please use Matlab to solve this question:
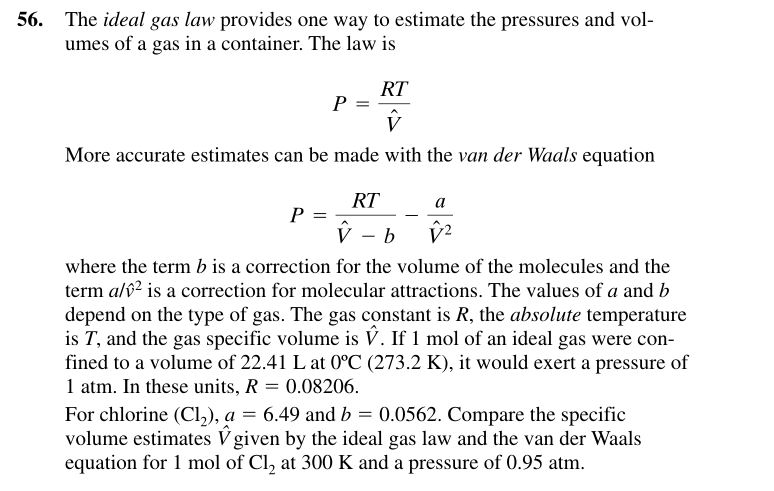
56. The ideal gas law provides one way to estimate the pressures and vol- umes of a gas in a container. The law is RT More accurate estimates can be made with the van der Waals equation RT a where the term b is a correction for the volume of the molecules and the term al02 is a correction for molecular attractions. The values of a and b depend on the type of gas. The gas constant is R, the absolute temperature is T, and the gas specific volume is V. If 1 mol of an ideal gas were con fined to a volume of 22.41 L at 0C (273.2 K), it would exert a pressure of 1 atm. In these units, R 0.08206 For chlorine (C12), a = 6.49 and b 0.0562. Compare the specific volume estimates V given by the ideal gas law and the van der Waals equation for 1 mol of Cl2 at 300 K and a pressure of 0.95 atm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


