Question: please use method shown in first picture This graph shows a plot of concentration versus time for a o.10o M solution of C12H22O11. The reaction
please use method shown in first picture
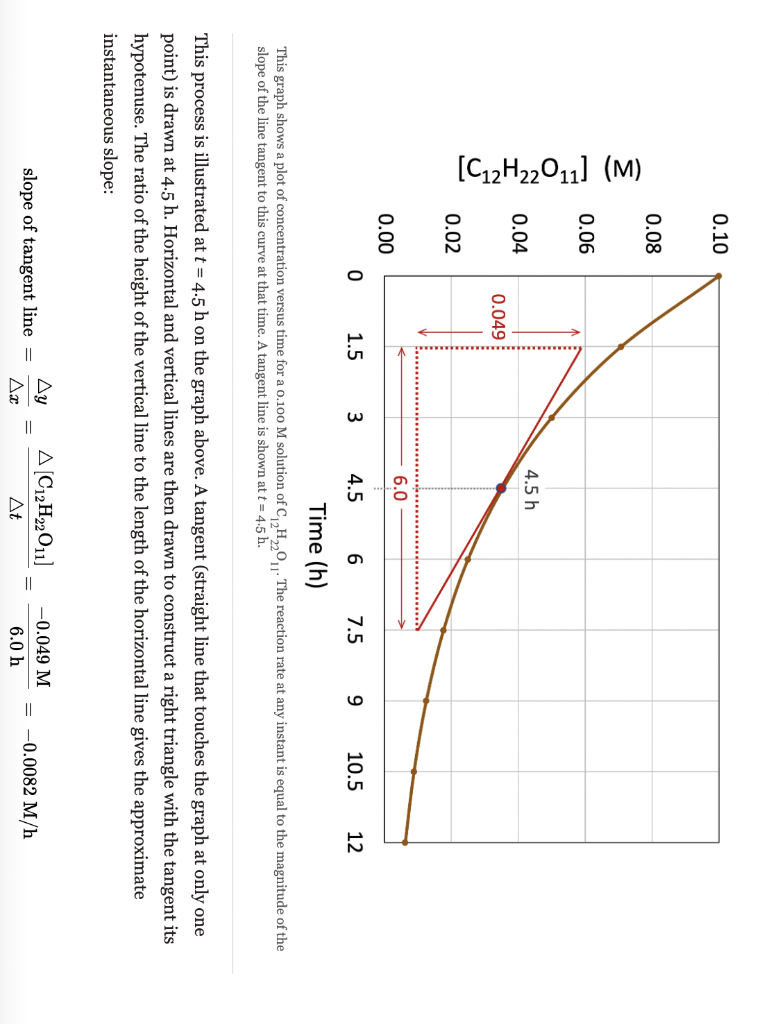
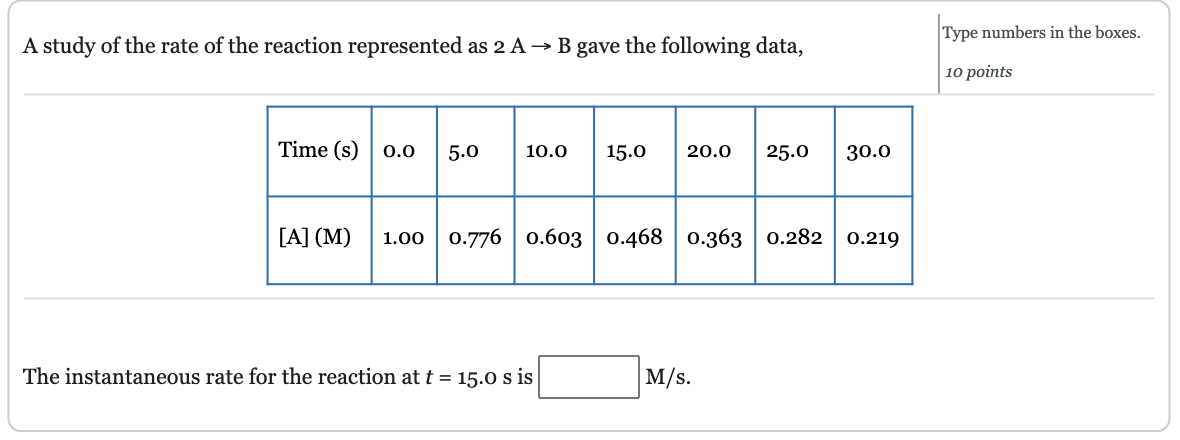
This graph shows a plot of concentration versus time for a o.10o M solution of C12H22O11. The reaction rate at any instant is equal to the magnitude of the slope of the line tangent to this curve at that time. A tangent line is shown at t=4.5h. This process is illustrated at t=4.5h on the graph above. A tangent (straight line that touches the graph at only one point) is drawn at 4.5h. Horizontal and vertical lines are then drawn to construct a right triangle with the tangent its hypotenuse. The ratio of the height of the vertical line to the length of the horizontal line gives the approximate instantaneous slope: slopeoftangentline=xy=t[C12H22O11]=6.0h0.049M=0.0082M/h A study of the rate of the reaction represented as 2AB gave the following data, Type numbers in the boxes. 1o points The instantaneous rate for the reaction at t=15.0s is M/s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


