Question: please use simple code blocks code The Ideal Gas law allows us to know the volume V (liters) that a quantity n(moles) of a gas
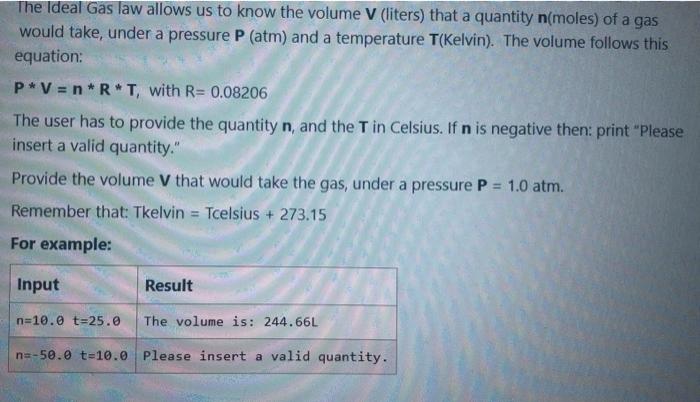
The Ideal Gas law allows us to know the volume V (liters) that a quantity n(moles) of a gas would take, under a pressure P (atm) and a temperature T(Kelvin). The volume follows this equation: P*V = n*R*T, with R= 0.08206 The user has to provide the quantity n, and the T in Celsius. If n is negative then: print "Please insert a valid quantity." Provide the volume V that would take the gas, under a pressure P = 1.0 atm. Remember that: Tkelvin = Tcelsius + 273.15 For example: Input Result n=10.0 t=25.0 The volume is: 244.66L na-50.0 t=10.0 Please insert a valid quantity
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


