Question: pls answer this asap Use the References to access important values if needed for this question. For the following reaction, 28.0 grams of sulfur dioxide
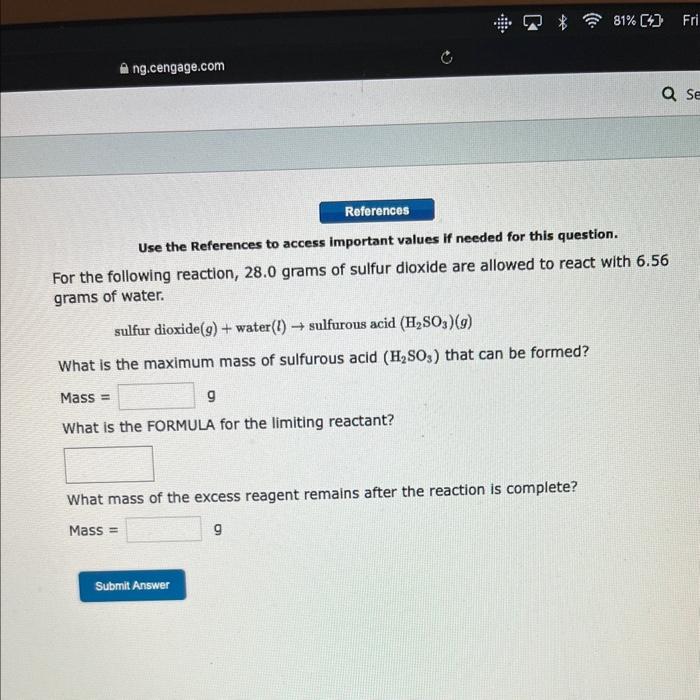
Use the References to access important values if needed for this question. For the following reaction, 28.0 grams of sulfur dioxide are allowed to react with 6.56 grams of water. sulfur dioxide (g)+ water (l) sulfurous acid (H2SO3)(g) What is the maximum mass of sulfurous acid (H2SO3) that can be formed? Mass = 9 What is the FORMULA for the limiting reactant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


