Question: points out of 5) For the 3 parallel equal-volume reactors shown in the figure below, in which the same reaction nor each which are run
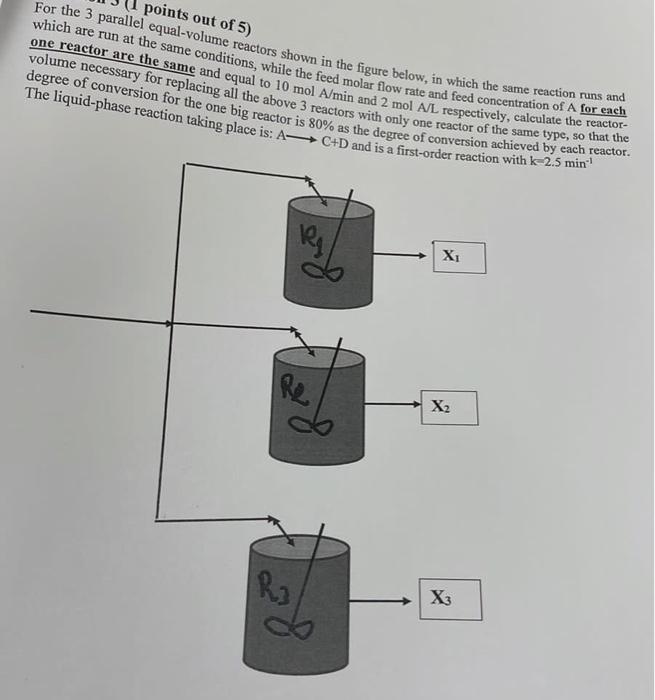
points out of 5) For the 3 parallel equal-volume reactors shown in the figure below, in which the same reaction nor each which are run at the same conditions, while the feed molar flow rate and feed concentration of A for each one reactor are the same and equal to 10 mol A/min and 2 mol A/L respectively, calculate the reactor- volume necessary for replacing all the above 3 reactors with only one reactor of the same type, so that the degree of conversion for the one big reactor is 80% as the degree of conversion achieved by each reactor. The liquid-phase reaction taking place is: A-C+D and is a first-order reaction with k-2.5 min X f Re X2 R3 X3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


