Question: Problem 1 : There is a single first - order reaction A B , taking place in a reactor. The rate of change of concentration
Problem : There is a single firstorder reaction taking place in a reactor. The rate of change of concentration with time is given as follows;
Here is the concentration of is the reaction rate constant and is time in minutes. The reactor data are given in the following table;
tableTime min,kmo
Use finite differencing and the linear least squares regression to find the reaction rate constant.
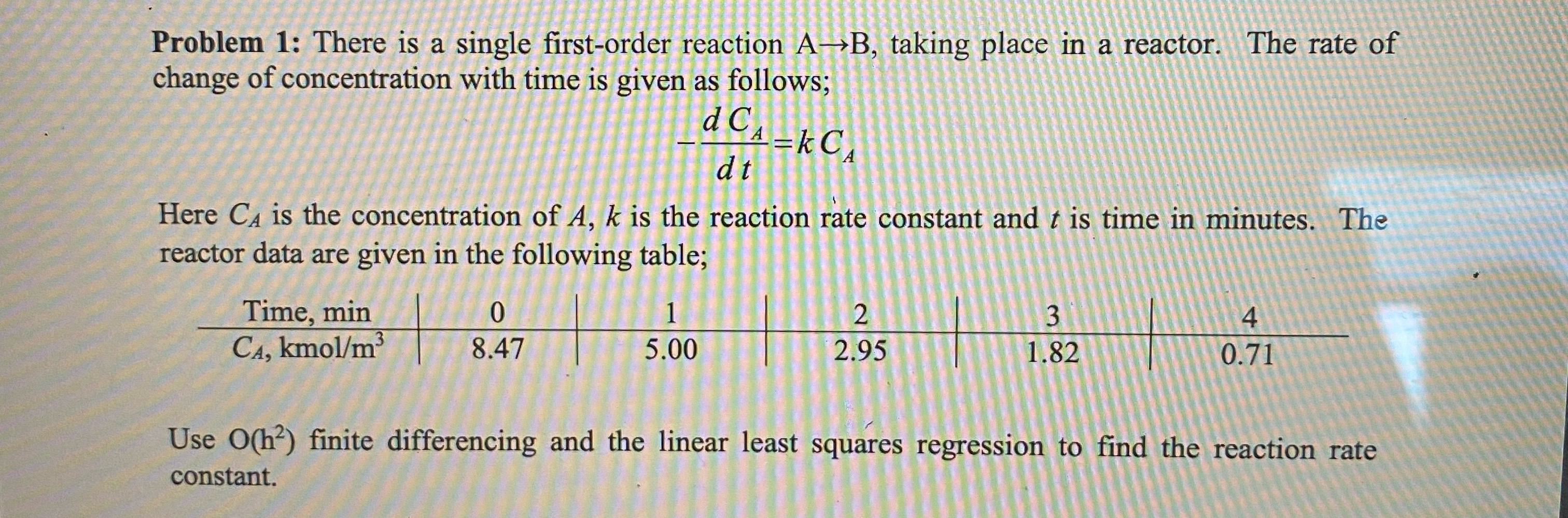
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


