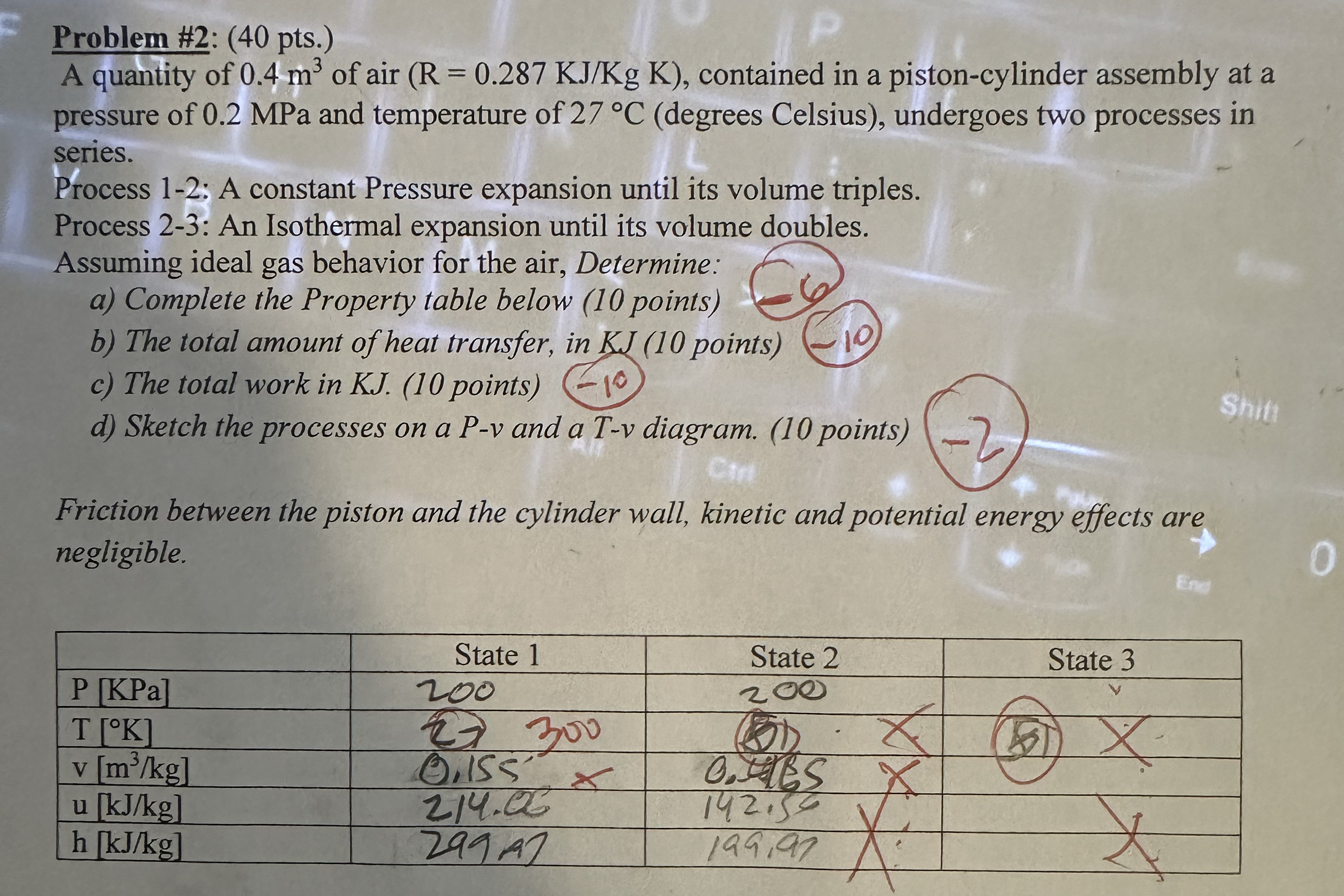
Question: Problem # 2 : ( 4 0 pts . ) A quantity of 0 . 4 m 3 of air ( R = 0 .
Problem #: pts
A quantity of of air contained in a pistoncylinder assembly at a pressure of MPa and temperature of degrees Celsius undergoes two processes in series.
Process ; A constant Pressure expansion until its volume triples.
Process : An Isothermal expansion until its volume doubles.
Assuming ideal gas behavior for the air, Determine:
a Complete the Property table below points
b The total amount of heat transfer, in KJ points
c The total work in KJ points
d Sketch the processes on a and a Tv diagram. points
Shith
Friction between the piston and the cylinder wall, kinetic and potential energy effects are negligible.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


