Question: Problem 2 (adapted from P2-5, textbook, Chapter 2) We want to formulate a reactor arrangement for the irreversible gas-phase reaction A B This reaction is
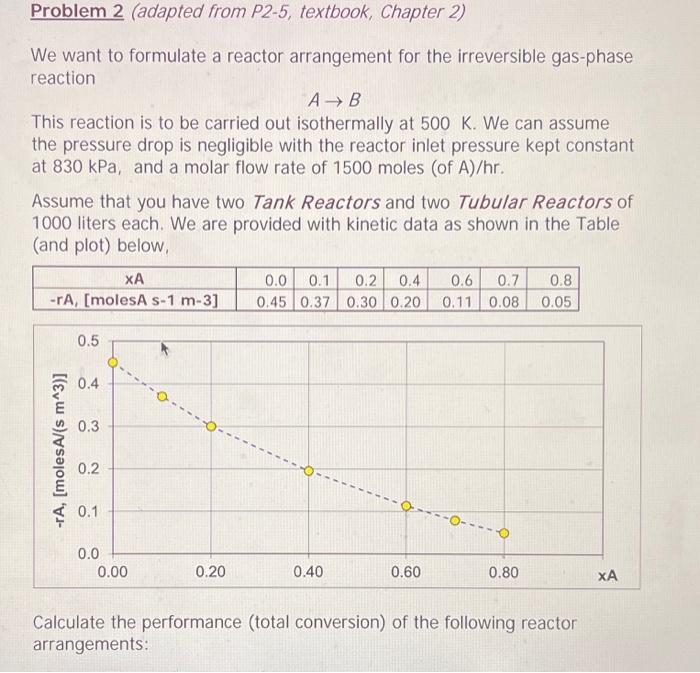

Problem 2 (adapted from P2-5, textbook, Chapter 2) We want to formulate a reactor arrangement for the irreversible gas-phase reaction A B This reaction is to be carried out isothermally at 500 K. We can assume the pressure drop is negligible with the reactor inlet pressure kept constant at 830 kPa, and a molar flow rate of 1500 moles (of A)/hr. Assume that you have two Tank Reactors and two Tubular Reactors of 1000 liters each. We are provided with kinetic data as shown in the Table (and plot) below, 0.8 XA -TA, [molesA s-1 m-3] 0.0 0.1 0.2 0.4 0.45 0.37 0.30 0.20 0.6 0.7 0.11 0.08 0.05 0.5 0.4 0.3 -TA, [molesAl(s m^3)] 0.2 $ 0.1 0.0 0.00 0.20 0.40 0.60 0.80 XA Calculate the performance (total conversion) of the following reactor arrangements: A single Tubular Reactor (b) A sequence with the two Tubular Reactors in series (c) An arrangement with the two Tubular Reactors in parallel with the feed (F) divided equally between the two reactors. (d) A three-reactor-sequence of one Tubular Reactor followed by a Tank Reactor, followed by a Tubular Reactor
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


