Question: Problem 3 : Butane and oxygen combine in the combustion process. Balance the following chemical equation that describes the combustion of butane: C 4 H
Problem:
Butane and oxygen combine in the combustion process. Balance the following chemical equation that describes the combustion of butane:
a How many moles of oxygen are required to burn mol of butane?
b How many grams of oxygen are required to burn of butane?
c At standard temperature and pressure, how many moles of oxygen would be required to burn of butane?
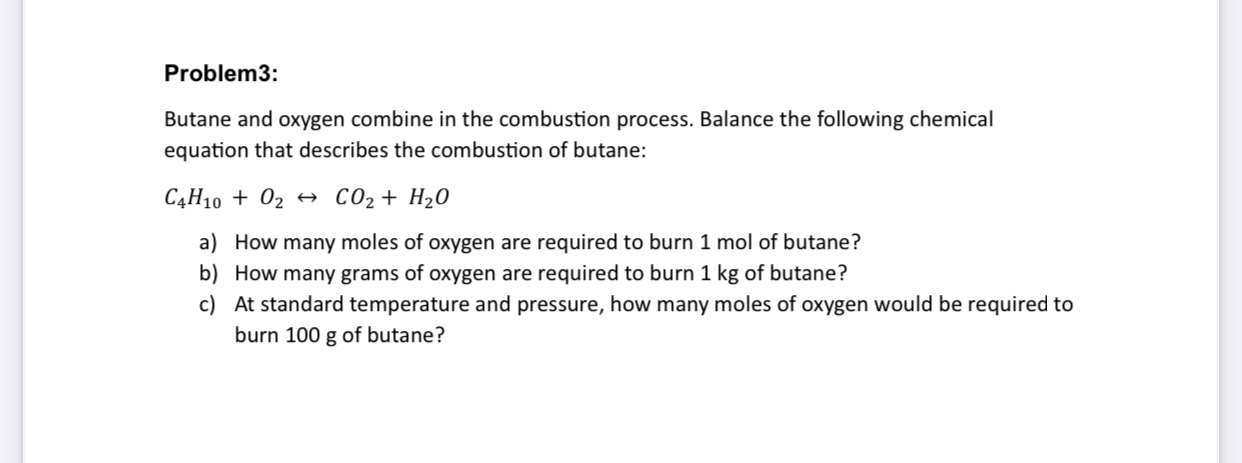
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


