Question: Problem 4 1500 kg of reactant A (MW = 200 kg/kmol) is added to 5500 kg of a mixture of organic solvent (MW =
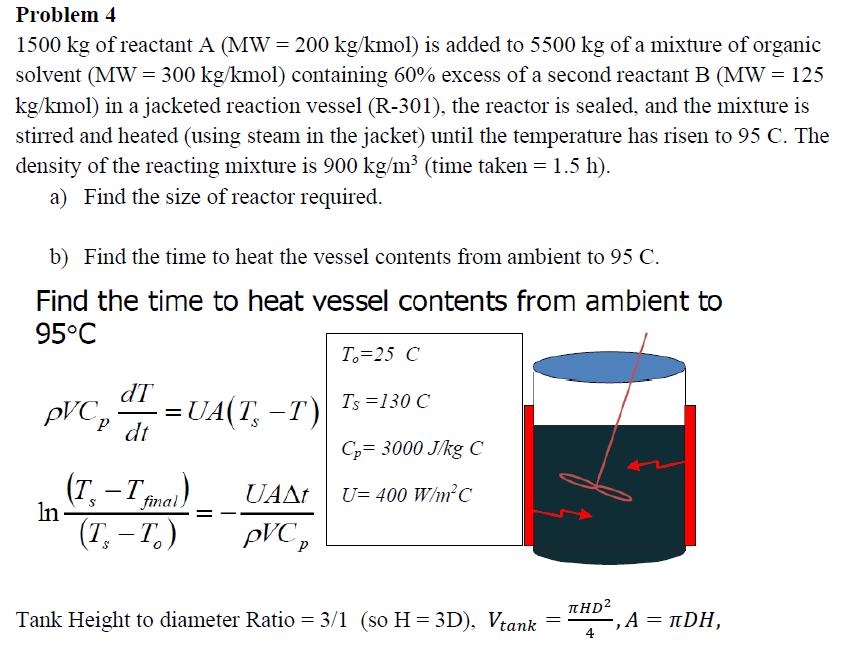
Problem 4 1500 kg of reactant A (MW = 200 kg/kmol) is added to 5500 kg of a mixture of organic solvent (MW = 300 kg/kmol) containing 60% excess of a second reactant B (MW = 125 kg/kmol) in a jacketed reaction vessel (R-301), the reactor is sealed, and the mixture is stirred and heated (using steam in the jacket) until the temperature has risen to 95 C. The density of the reacting mixture is 900 kg/m (time taken = 1.5 h). a) Find the size of reactor required. b) Find the time to heat the vessel contents from ambient to 95 C. Find the time to heat vessel contents from ambient to 95C dT PVC p dt In (T T-T =UA(T-T) final, (T. -T.) UAAt PVC p T. 25 C Ts=130 C Cp= 3000 J/kg C U= 400 W/mC Tank Height to diameter Ratio = 3/1 (so H=3D), Vtank HD = -, A = DH,
Step by Step Solution
There are 3 Steps involved in it
given that 13 C 900 kgm t 15m 1SX 3600 t5400 Secs To 25C Ts 130C Gp 3000 Jkgt a To ... View full answer

Get step-by-step solutions from verified subject matter experts


