Question: Problem 4. The parallel first order catalytic reactions: A-B A C take place in a tube packed with spherical catalyst pellets with radius 3
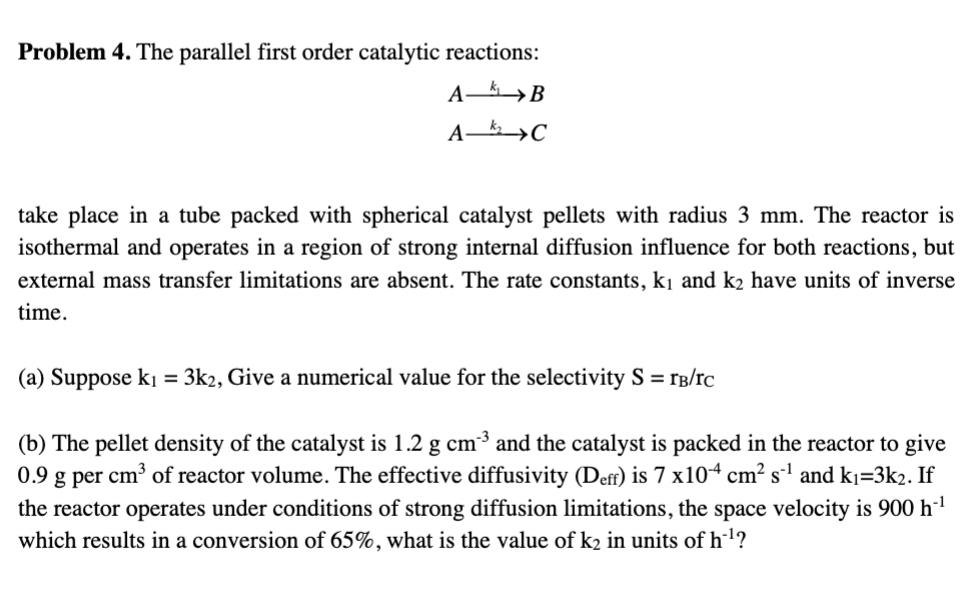
Problem 4. The parallel first order catalytic reactions: A-B A C take place in a tube packed with spherical catalyst pellets with radius 3 mm. The reactor is isothermal and operates in a region of strong internal diffusion influence for both reactions, but external mass transfer limitations are absent. The rate constants, k and k have units of inverse time. (a) Suppose k = 3k2, Give a numerical value for the selectivity S = B/TC (b) The pellet density of the catalyst is 1.2 g cm and the catalyst is packed in the reactor to give 0.9 g per cm of reactor volume. The effective diffusivity (Deff) is 7 x104 cm s and k=3k2. If the reactor operates under conditions of strong diffusion limitations, the space velocity is 900 h* which results in a conversion of 65%, what is the value of k in units of h?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


