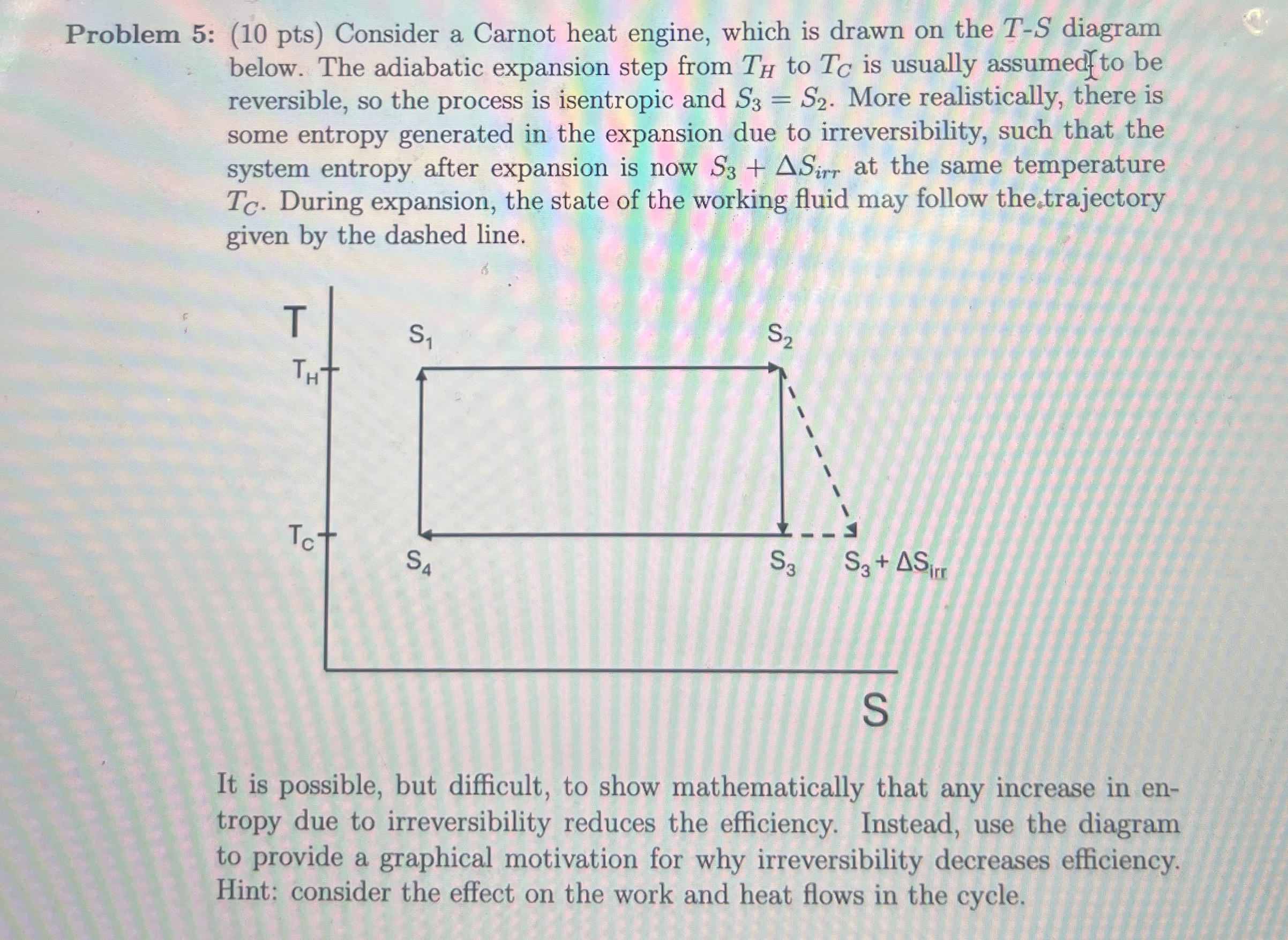
Question: Problem 5 : ( 1 0 pts ) Consider a Carnot heat engine, which is drawn on the T - S diagram below. The adiabatic
Problem : pts Consider a Carnot heat engine, which is drawn on the diagram below. The adiabatic expansion step from to is usually assumedfto be reversible, so the process is isentropic and More realistically, there is some entropy generated in the expansion due to irreversibility, such that the system entropy after expansion is now at the same temperature During expansion, the state of the working fluid may follow the trajectory given by the dashed line.
It is possible, but difficult, to show mathematically that any increase in entropy due to irreversibility reduces the efficiency. Instead, use the diagram to provide a graphical motivation for why irreversibility decreases efficiency. Hint: consider the effect on the work and heat flows in the cycle.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


