Question: Problem 7-5 (Level 2) The homogeneous, gas-phase reactions A+B+C B+D+E are taking place in an ideal, continuous stirred-tank reactor. The temperature is 400 K and
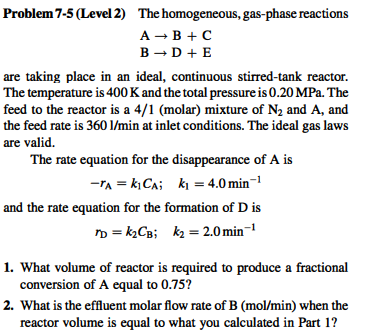
Problem 7-5 (Level 2) The homogeneous, gas-phase reactions A+B+C B+D+E are taking place in an ideal, continuous stirred-tank reactor. The temperature is 400 K and the total pressure is 0.20 MPa. The feed to the reactor is a 4/1 (molar) mixture of N, and A, and the feed rate is 360 1/min at inlet conditions. The ideal gas laws are valid. The rate equation for the disappearance of A is -"^ = k1CA; k, = 4.0 min-1 and the rate equation for the formation of Dis no =kCB; kz = 2.0 min-1 1. What volume of reactor is required to produce a fractional conversion of A equal to 0.75? 2. What is the effluent molar flow rate of B (mol/min) when the reactor volume is equal to what you calculated in
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


