Question: Process Description The process to be modeled is part of the production of methanol from Natural Gas. It is assumed that the Natural Gas is
Process Description The process to be modeled is part of the production of methanol from Natural Gas. It is assumed that the Natural Gas is already converted to Synthesis Gas (Syngas) with the following composition and conditions:
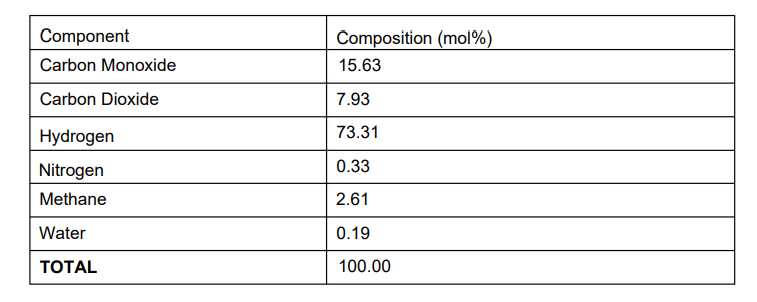 Temperature 131.7 C Pressure 1187 psia The flow of the Syngas stream is 1000 kgmole/hr. The feed stream is mixed with the recycle and heated to a temperature of 130 C, and the pressure drop across this exchanger is assumed to be zero. This stream is fed to the reactor which performs the following two equilibrium reactions: CO2 + 3 H2 CH3OH + H2O and CO + 2 H2 CH3OH Note that both of these reactions are reversible, and the overall reaction is exothermic. For Keq you can select Gibbs Free Energy option. Note that there is a pressure drop of 186 kPa across this reactor. The reactor effluent is cooled to a temperature of 37.78 C in a cooler which has a pressure drop of 110.3 kPa. This stream is then flashed to give vapour and liquid streams. The pressure drop across this flash separator is assumed to be zero. The vapour stream is sent to the recycle, but before this a purge stream is taken off. This purge stream is set at 4.8% of the vapour stream from the flash. After this purge stream is taken off, the recycle stream is compressed to 8190 kPa before being returned to mix with the feed. The recycle compressor has an adiabatic efficiency of 70% The liquid stream from the first flash is sent to a second flash separator which has a pressure drop of 7587 kPa to produce a liquid crude methanol product and a vapour stream which is sent as loop flash gas to the fuel system. The methanol specification requires a minimum purity of 75 mol%. QUESTIONS (a) Supply a process flow diagram from the process description given and give appropriate names to each of the streams (hand drawn). (b) When you have found a converged solution at Base Case Conditions, calculate the mass balance errors. For each and every component in the process, complete the Table 1 below.
Temperature 131.7 C Pressure 1187 psia The flow of the Syngas stream is 1000 kgmole/hr. The feed stream is mixed with the recycle and heated to a temperature of 130 C, and the pressure drop across this exchanger is assumed to be zero. This stream is fed to the reactor which performs the following two equilibrium reactions: CO2 + 3 H2 CH3OH + H2O and CO + 2 H2 CH3OH Note that both of these reactions are reversible, and the overall reaction is exothermic. For Keq you can select Gibbs Free Energy option. Note that there is a pressure drop of 186 kPa across this reactor. The reactor effluent is cooled to a temperature of 37.78 C in a cooler which has a pressure drop of 110.3 kPa. This stream is then flashed to give vapour and liquid streams. The pressure drop across this flash separator is assumed to be zero. The vapour stream is sent to the recycle, but before this a purge stream is taken off. This purge stream is set at 4.8% of the vapour stream from the flash. After this purge stream is taken off, the recycle stream is compressed to 8190 kPa before being returned to mix with the feed. The recycle compressor has an adiabatic efficiency of 70% The liquid stream from the first flash is sent to a second flash separator which has a pressure drop of 7587 kPa to produce a liquid crude methanol product and a vapour stream which is sent as loop flash gas to the fuel system. The methanol specification requires a minimum purity of 75 mol%. QUESTIONS (a) Supply a process flow diagram from the process description given and give appropriate names to each of the streams (hand drawn). (b) When you have found a converged solution at Base Case Conditions, calculate the mass balance errors. For each and every component in the process, complete the Table 1 below. 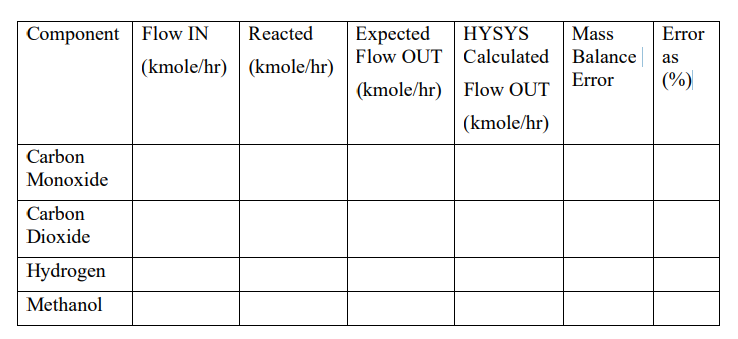 (c) Hysys file at Base Case Conditions. (d) Using HYSYS Case Study tool draw graphs to show how the following vary as the purge ratio is changed from 2% to 10%: - hydrogen molar flow in purge stream - crude methanol production rate - methanol purity (e) Using HYSYS Case Study tool draw graphs to show how the following vary as the reactor inlet temperature is changed from 120 to 150 C: (1) CO conversion rate (2) CO2 conversion rate (3) crude methanol production rate
(c) Hysys file at Base Case Conditions. (d) Using HYSYS Case Study tool draw graphs to show how the following vary as the purge ratio is changed from 2% to 10%: - hydrogen molar flow in purge stream - crude methanol production rate - methanol purity (e) Using HYSYS Case Study tool draw graphs to show how the following vary as the reactor inlet temperature is changed from 120 to 150 C: (1) CO conversion rate (2) CO2 conversion rate (3) crude methanol production rate
\begin{tabular}{|l|l|} \hline Component & Composition (mol\%) \\ \hline Carbon Monoxide & 15.63 \\ \hline Carbon Dioxide & 7.93 \\ \hline Hydrogen & 73.31 \\ \hline Nitrogen & 0.33 \\ \hline Methane & 2.61 \\ \hline Water & 0.19 \\ \hline TOTAL & 100.00 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


