Question: Provide the condensed electron configuration for Silver ( Ag has a filled d subshell). Format for entering your answer: - The electronic configuration for O
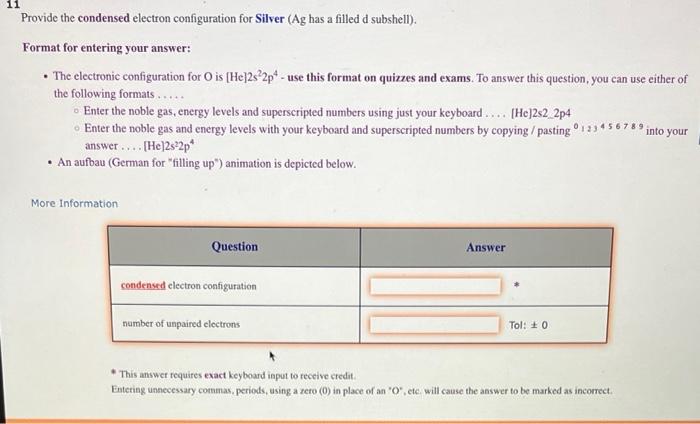
Provide the condensed electron configuration for Silver ( Ag has a filled d subshell). Format for entering your answer: - The electronic configuration for O is [He] 2s22p4 - use this format on quizzes and exams. To answer this question, you can use either of the following formats..... Enter the noble gas, energy levels and superscripted numbers using just your keyboard.... [He]2s2 2p4 Enter the noble gas and energy levels with your keyboard and superscripted numbers by copying / pasting 0123456789 into your answer.... [He]2s 22p4 - An aufbau (German for "filling up") animation is depicted below. More information * This answer requires exact keyboard iaput to receive credit. Euteriag unnecessary conmmas, periods, using a zero (0) in place of an ' 0 ', ete. will cause the answer to be marked as incorrect
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


