Question: Q 3.9 ) is an important chemical intermediate. When using the pure 2-butanone ( liquid compound as reference state, in which solvents (give examples) would

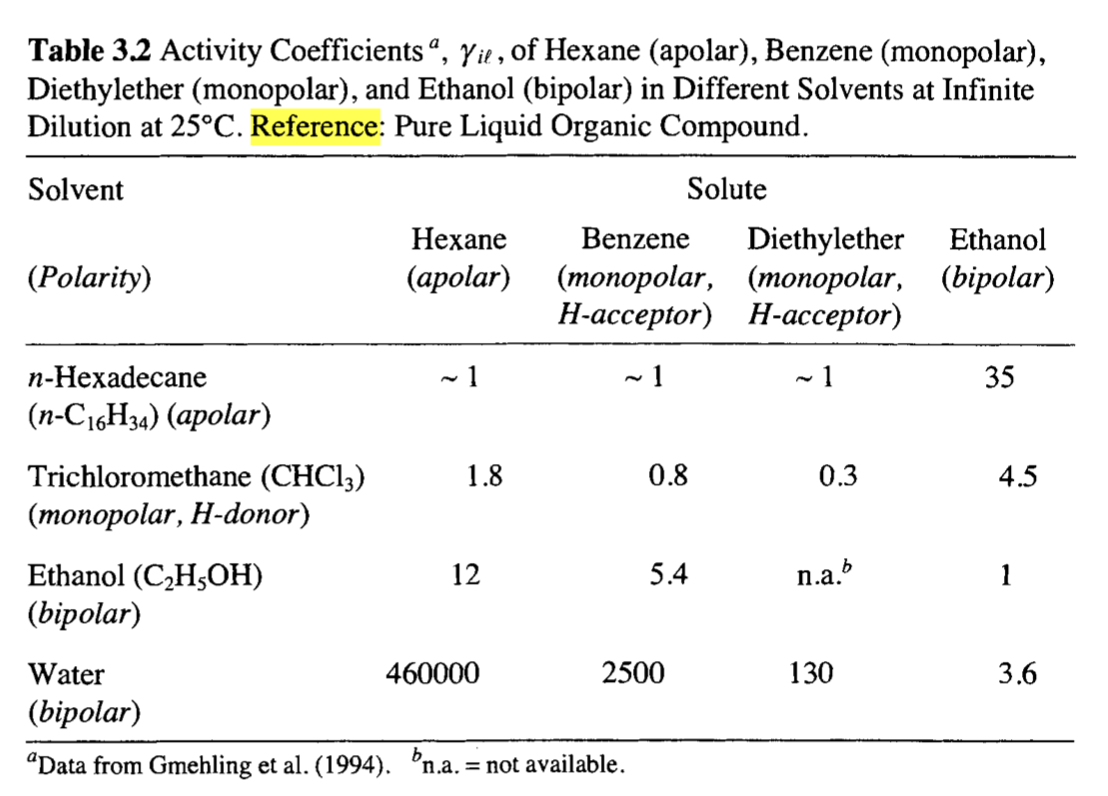
Q 3.9 ) is an important chemical intermediate. When using the pure 2-butanone ( liquid compound as reference state, in which solvents (give examples) would you expect that this compound has an activity coefficient of (a) close to 1, (b) smaller than one, and (c) larger than one? (Table 3.2 might be helpful.) 9 Table 3.2 Activity Coefficients, Yil, of Hexane (apolar), Benzene (monopolar), Diethylether (monopolar), and Ethanol (bipolar) in Different Solvents at Infinite Dilution at 25C. Reference: Pure Liquid Organic Compound. Solvent Hexane (apolar) Solute Benzene Diethylether Ethanol (monopolar, (monopolar, (bipolar) H-acceptor) H-acceptor) (Polarity) ~ 1 ~1 ~ 1 35 n-Hexadecane (n-C16H34) (apolar) 0.3 4.5 n.a. 1 Trichloromethane (CHCI3) 1.8 0.8 (monopolar, H-donor) Ethanol (C2H5OH) 12 5.4 (bipolar) Water 460000 2500 (bipolar) "Data from Gmehling et al. (1994). bn.a. = not available. 130 3.6
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


