Question: Q = 9 N1 1 9 = qEqV9TAR T IT 2 T3 1 qR,diamtomic ; qR,polyatomic OAO BOC : qv modes 1 - exp (-
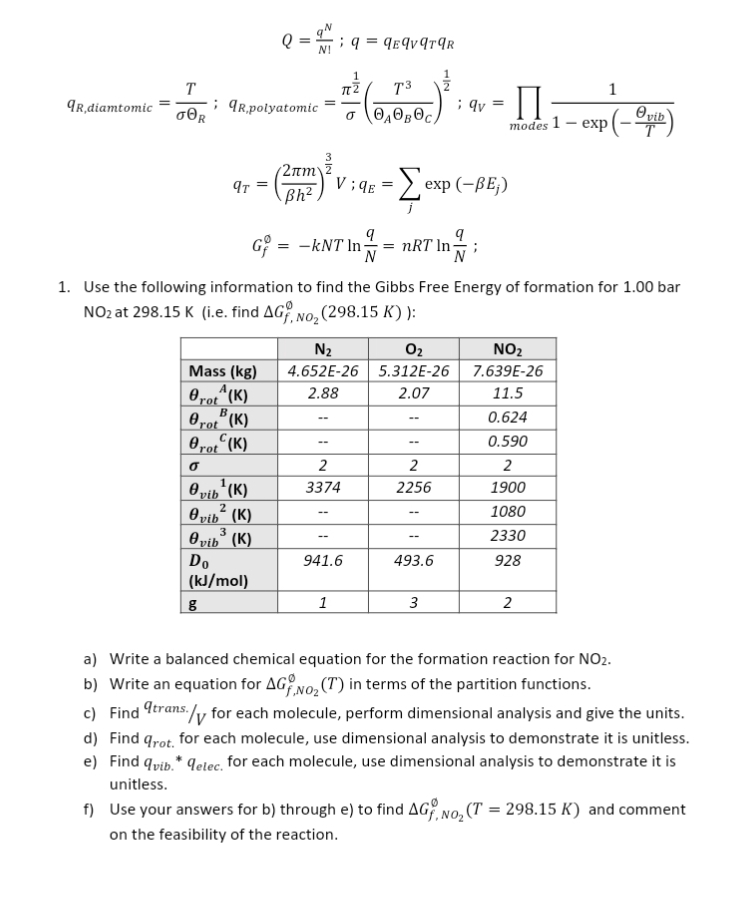
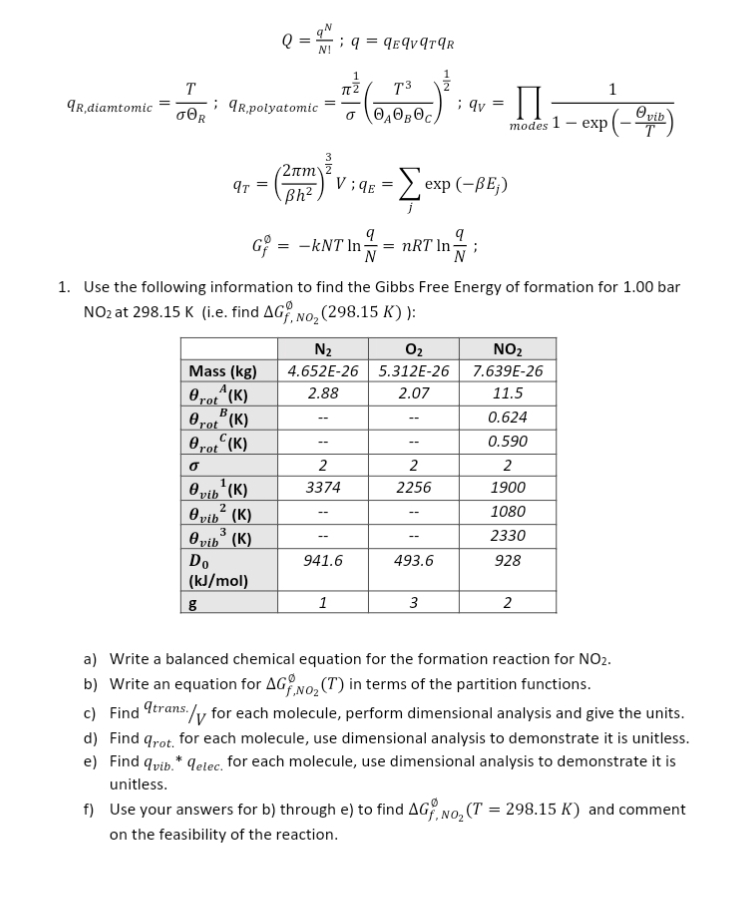
Q = 9" N1 1 9 = qEqV9TAR T IT 2 T3 1 qR,diamtomic ; qR,polyatomic OAO BOC : qv modes 1 - exp (- e vib 2um qT = Bh z Lexp (-BE;) G? = -kNT In = nRT InN ; 1. Use the following information to find the Gibbs Free Energy of formation for 1.00 bar NO2 at 298.15 K (i.e. find AG, No2 (298.15 K) ): N2 Oz NO2 Mass (kg) 4.652E-26 5.312E-26 7.639E-26 Brot (K) 2.88 2.07 11.5 Brot (K) 0.624 Brot (K) -- 0.590 2 2 2 vib (K) 3374 2256 1900 @ vib (K) 1080 O vib (K) 2330 Do 941.6 493.6 928 (kJ/mol) g 1 3 2 a) Write a balanced chemical equation for the formation reaction for NO2. b) Write an equation for AG No2 (T) in terms of the partition functions. c) Find trans./ for each molecule, perform dimensional analysis and give the units. d) Find qrot. for each molecule, use dimensional analysis to demonstrate it is unitless. e) Find quib. * qelec. for each molecule, use dimensional analysis to demonstrate it is unitless. f) Use your answers for b) through e) to find AG, NO, (T = 298.15 K) and comment on the feasibility of the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


