Question: Q.2. (8 marks) Consider the process shown in the figure below, where carbon monoxide (CO) gas is being oxidized to carbon dioxide (CO2). This process
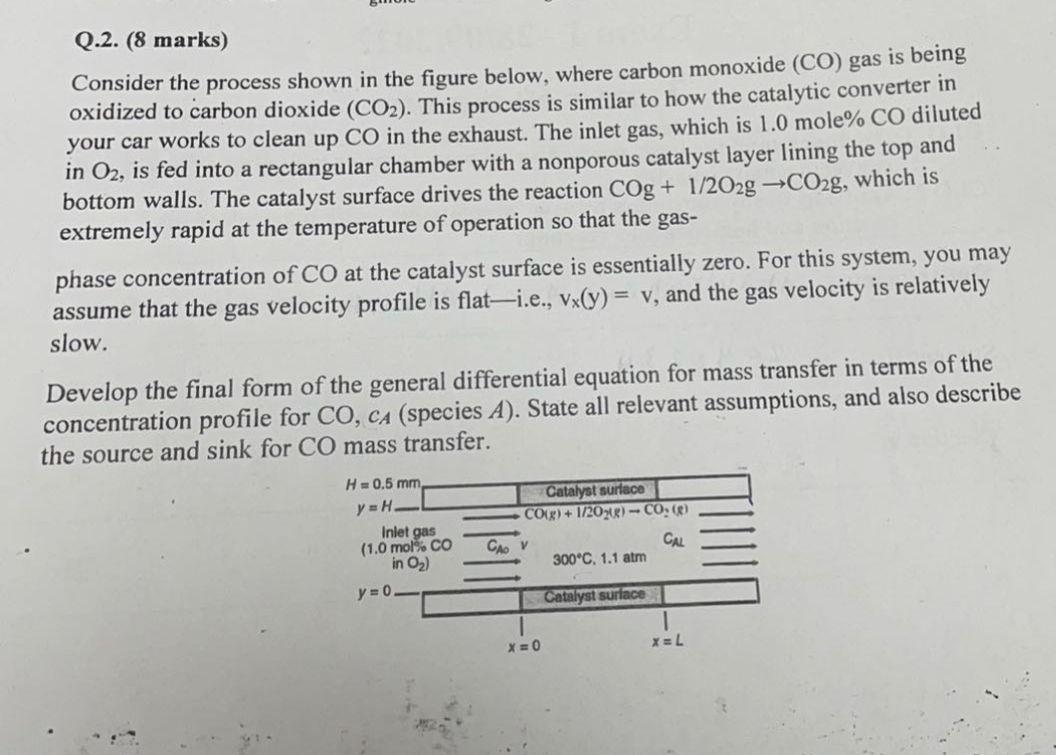
Q.2. (8 marks) Consider the process shown in the figure below, where carbon monoxide (CO) gas is being oxidized to carbon dioxide (CO2). This process is similar to how the catalytic converter in your car works to clean up CO in the exhaust. The inlet gas, which is 1.0mole%CO diluted in O2, is fed into a rectangular chamber with a nonporous catalyst layer lining the top and bottom walls. The catalyst surface drives the reaction COg+1/2O2gCO2g, which is extremely rapid at the temperature of operation so that the gas- phase concentration of CO at the catalyst surface is essentially zero. For this system, you may assume that the gas velocity profile is flat-i.e., vx(y)=vy, and the gas velocity is relatively slow. Develop the final form of the general differential equation for mass transfer in terms of the concentration profile for CO,cA (species A ). State all relevant assumptions, and also describe the source and sink for CO mass transfer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


