Question: Q7. A compound with empirical formula Co(NH),BrSO exist in two forms red and violet. Solution of red colour gives a precipitate of AgBr on
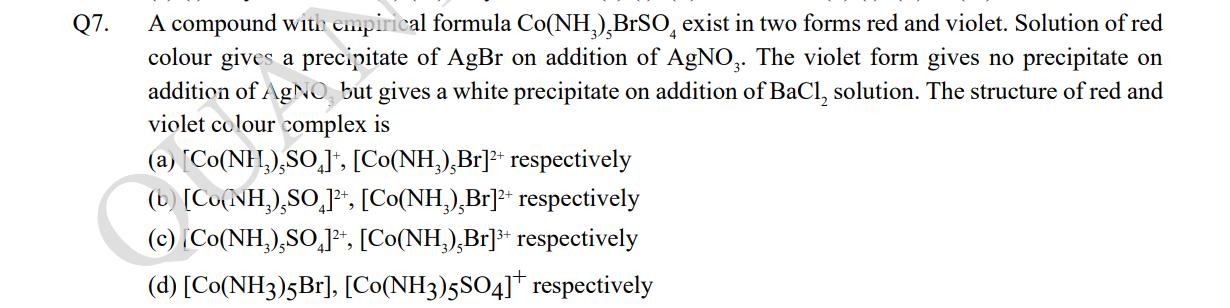
Q7. A compound with empirical formula Co(NH),BrSO exist in two forms red and violet. Solution of red colour gives a precipitate of AgBr on addition of AgNO3. The violet form gives no precipitate on addition of AgNO, but gives a white precipitate on addition of BaCl, solution. The structure of red and violet colour complex is (a) [Co(NH),SO], [Co(NH3),Br]+ respectively (b) [Co(NH),SO 1, [Co(NH),Br]+ respectively (c) [Co(NH),SO]+, [Co(NH),Br]+ respectively (d) [Co(NH3)5Br], [Co(NH3)5SO4]* respectively
Step by Step Solution
3.40 Rating (162 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


