Question: Question 1 (a) (5 marks) Question 1 (c) (5 marks) The reaction A+B 2C takes place in an unsteady state flow reactor. The feed is
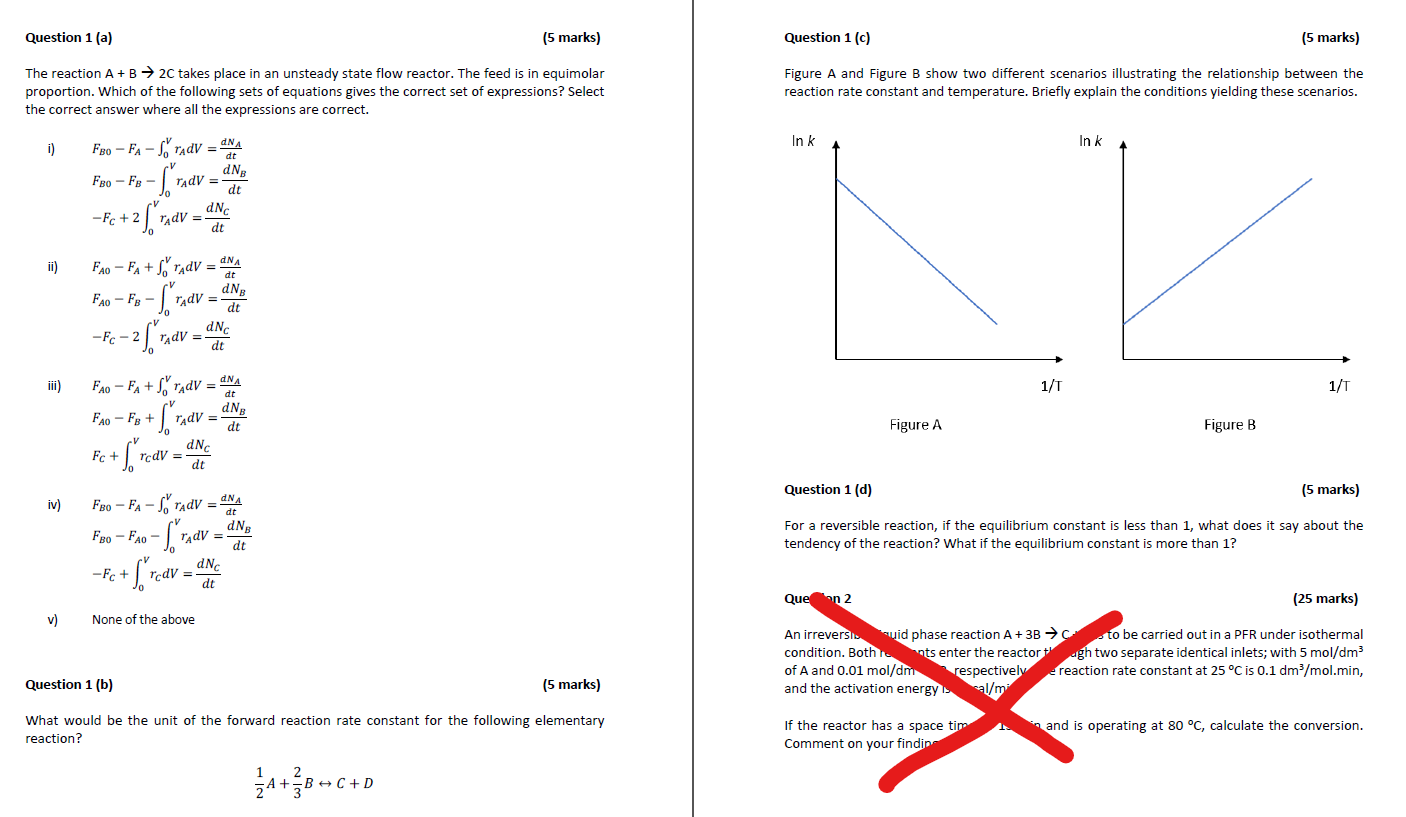
Question 1 (a) (5 marks) Question 1 (c) (5 marks) The reaction A+B 2C takes place in an unsteady state flow reactor. The feed is in equimolar proportion. Which of the following sets of equations gives the correct set of expressions? Select the correct answer where all the expressions are correct. Figure A and Figure B show two different scenarios illustrating the relationship between the reaction rate constant and temperature. Briefly explain the conditions yielding these scenarios. In k In k i) F80 - FA-ADV = F8o Fy - 5 dNC -Fc +2 2 STAV = V ridV = dNA dt dNB dt = dt ii) dt FAO - FA+S"rady = NA STAV Fao F - $ San AdV dN -Fc-2 dNC dt V - 21 AdV = dt iii) 1/1 1/T dt = +V - o $" NA FAO - FA+STAV = dNg FAO - Fg + ridV dt dNC Fc + rcdV = dt Figure A Figure B Question 1 (d) (5 marks) iv) = = DNA F80 - FA - So'radV = dt dNg FBo - FAO - rAdV = dt Nc -Fc + rcdV dt For a reversible reaction, if the equilibrium constant is less than 1, what does it say about the tendency of the reaction? What if the equilibrium constant is more than 1? S + = - Que on 2 (25 marks) v) None of the above An irreversi.uid phase reaction A +3B to be carried out in a PFR under isothermal condition. Both.nts enter the reactor ugh two separate identical inlets; with 5 mol/dm3 of A and 0.01 mol/dm respectively reaction rate constant at 25C is 0.1 dm/mol.min, and the activation energy. l/m Question 1 (b) (5 marks) What would be the unit of the forward reaction rate constant for the following elementary reaction? If the reactor has a space tim Comment on your findin and is operating at 80 C, calculate the conversion. 1 A+B+C+D 2 B + 34
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


