Question: Question 1 Consider the following first-order ODE which represents the rate of formation of compound y: dy dt from t = 1 to t =
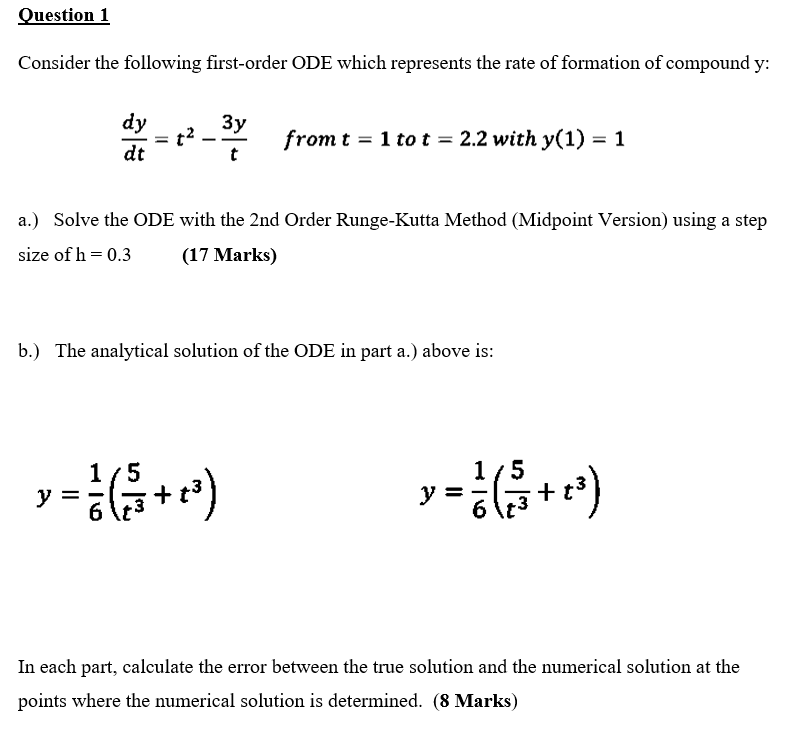
Question 1 Consider the following first-order ODE which represents the rate of formation of compound y: dy dt from t = 1 to t = 2.2 with y(1) = 1 t a.) Solve the ODE with the 2nd Order Runge-Kutta Method (Midpoint Version) using a step size of h=0.3 (17 Marks) b.) The analytical solution of the ODE in part a.) above is: 1/5 1/5 y = a +e) = y = S+) +t3 6 6 In each part, calculate the error between the true solution and the numerical solution at the points where the numerical solution is determined. (8 Marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


