Question: Given CH 3CO 2H(aq)=H(aq) + CH3CO 2 (aq) at 25C, Ka=1.83 x 10-5. What is A G at 25C for a solution in which
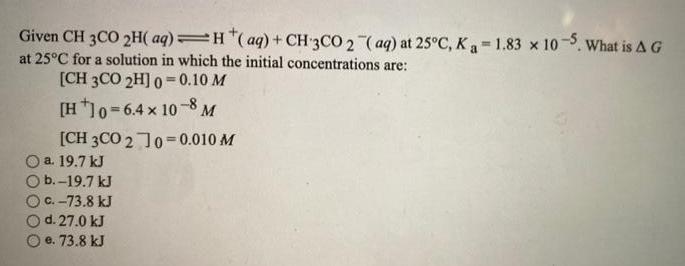
Given CH 3CO 2H(aq)=H(aq) + CH3CO 2 (aq) at 25C, Ka=1.83 x 10-5. What is A G at 25C for a solution in which the initial concentrations are: [CH 3CO 2H] 0 0.10 M [H +10=6.4 x 10-8 M [CH3CO 2 ]0=0.010 M a. 19.7 kJ b.-19.7 kJ 6. 73.8 kJ d. 27.0 kJ e. 73.8 kJ
Step by Step Solution
★★★★★
3.44 Rating (157 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Given that T 25c 298K 3D Ka 183 5 3D cngCoon o lo M 3D h 64 ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


