Question: Question 3 4 pts 12 ml Mg(OH)2 saturated solution with suitable indicator react with 10 ml HCl (0.3 M). Calculate Ksp, molar solubility of Mg(OH)2,
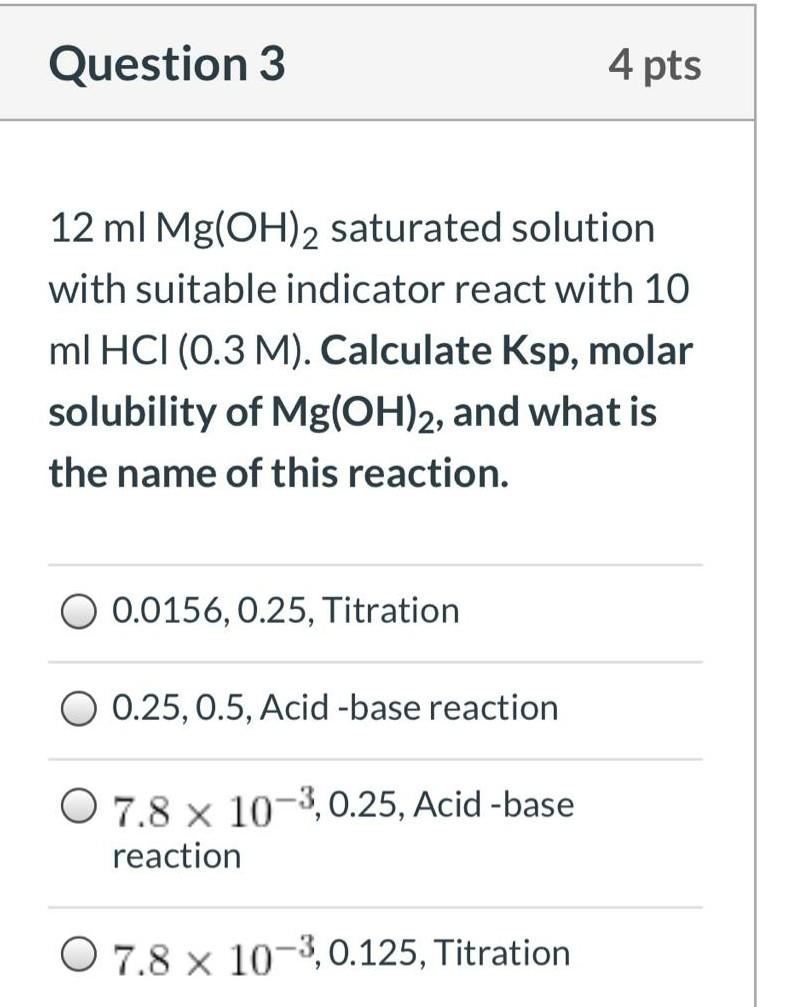
Question 3 4 pts 12 ml Mg(OH)2 saturated solution with suitable indicator react with 10 ml HCl (0.3 M). Calculate Ksp, molar solubility of Mg(OH)2, and what is the name of this reaction. 0 0.0156, 0.25, Titration 0.25, 0.5, Acid -base reaction O 7.8 x 10-3,0.25, Acid-base reaction O 7.8 x 10-3,0.125, Titration
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


