Question: Question 4 1 Paint Consider the titration curve, in which an acid is treated with aqueous 0.5 M NOOH. The pH at the equivalence point
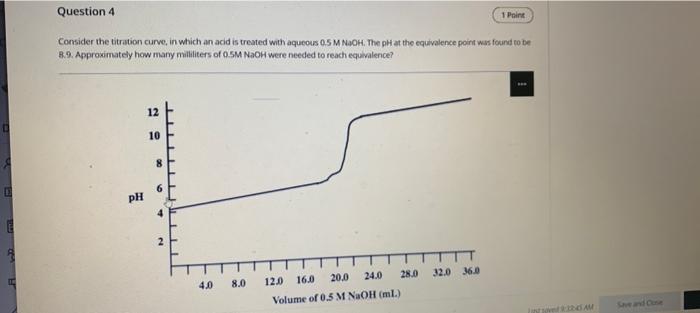
Question 4 1 Paint Consider the titration curve, in which an acid is treated with aqueous 0.5 M NOOH. The pH at the equivalence point was found to be 8.9. Approximately how many milliliters of 0.5M NaOH were needed to reach equivalence? 12 10 8 PH 4 2 TTT 32.0 36.0 4,0 8.0 12.0 16.0 20.0 24.0 28.0 Volume of 0.5 M NaOH (ml.) AM
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


