Question: Question 8 If the anode electrode in a voltaic cell is composed of a metal that participates in the oxidation half-cell reaction, what happens
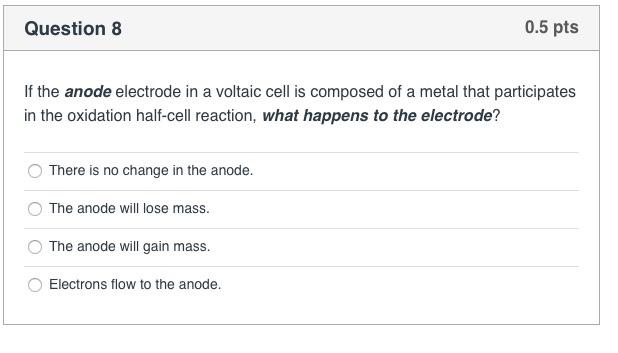
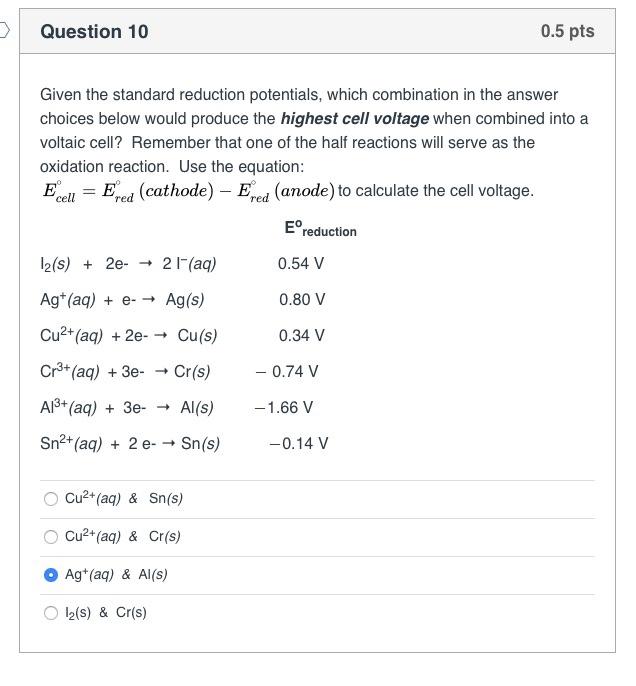
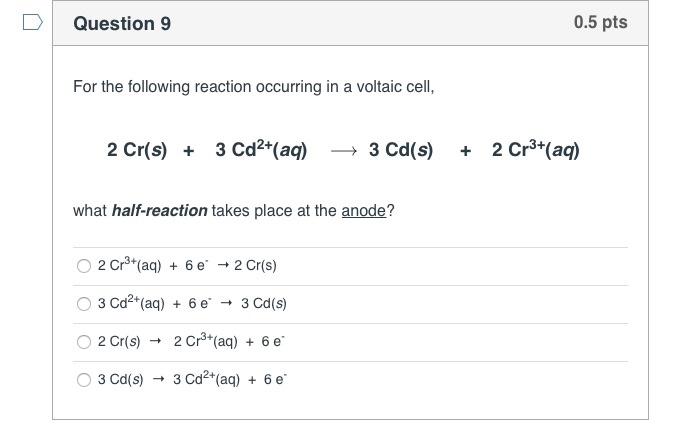

Question 8 If the anode electrode in a voltaic cell is composed of a metal that participates in the oxidation half-cell reaction, what happens to the electrode? There is no change in the anode. The anode will lose mass. The anode will gain mass. 0.5 pts Electrons flow to the anode. > Question 10 Given the standard reduction potentials, which combination in the answer choices below would produce the highest cell voltage when combined into a voltaic cell? Remember that one of the half reactions will serve as the oxidation reaction. Use the equation: E cell = = Ered Fred (cathode) - Ered (anode) to calculate the cell voltage. 12(s) + 2e- 21- (aq) Ag+ (aq) + e- Ag(s) Cu+ (aq) + 2e- Cu(s) Cr+ (aq) + 3e- Cr(s) Al+ (aq) + 3e- Al(s) Sn+ (aq) + 2 e- Sn(s) Cu2+ (aq) & Sn(s) Cu+ (aq) & Cr(s) Ag+ (aq) & Al(s) 12(S) & Cr(s) Ereduction 0.54 V 0.80 V 0.34 V - 0.74 V -1.66 V 0.5 pts -0.14 V Question 9 For the following reaction occurring in a voltaic cell, 2 Cr(s) + 3 Cd+ (aq) 3 Cd(s) what half-reaction takes place at the anode? 2 Cr+ (aq) + 6 e 2 Cr(s) 3 Cd+ (aq) + 6 e 3 Cd(s) 2 Cr(s) 2 Cr+ (aq) + 6 e 3 Cd(s) 3 Cd+ (aq) + 6 e 0.5 pts + 2 Cr+ (aq) >Question 7 The combination of the cathode reduction potential and the anode oxidation potential is known a the_ electron motive force (EMF) electron transfer quotient (ETQ) redox potential 0.5 pts electric current
Step by Step Solution
3.34 Rating (154 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


