Question: Required information Problem 0 7 . 1 0 0 - Using constant specific heat. A container filled with 5 5 . 0 kg of liquid
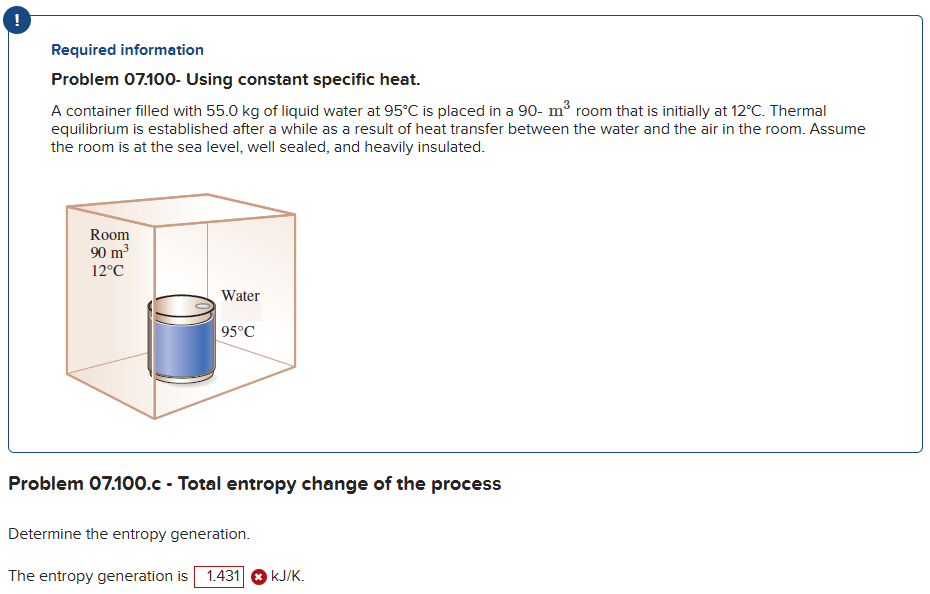
Required information
Problem Using constant specific heat.
A container filled with kg of liquid water at circmathrmC is placed in a mathrmm room that is initially at circmathrmC Thermal equilibrium is established after a while as a result of heat transfer between the water and the air in the room. Assume the room is at the sea level, well sealed, and heavily insulated.
Problem c Total entropy change of the process
Determine the entropy generation.
The entropy generation is boldsymbolmathrm~kJmathrmK
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


