Question: 9. Calculate the standard enthalpy (heat) of reaction for the oxidation of ammonia gas to produce nitrogen dioxide gas and water vapour given the
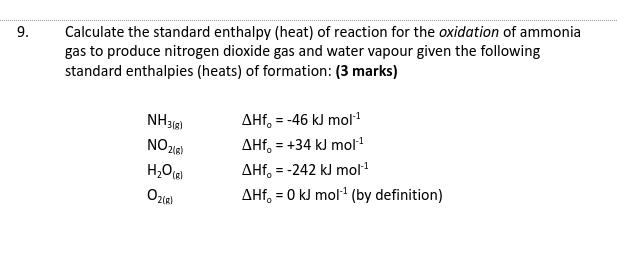
9. Calculate the standard enthalpy (heat) of reaction for the oxidation of ammonia gas to produce nitrogen dioxide gas and water vapour given the following standard enthalpies (heats) of formation: (3 marks) NH3(8) NO2(g) HO(g) O2(R) AHf, = -46 kJ mol AHf, +34 kJ mol- = AHf, = -242 kJ mol- AHf, = 0 kJ mol- (by definition)
Step by Step Solution
3.45 Rating (142 Votes )
There are 3 Steps involved in it
Oxidation of ammonia to and niterogen dioxide va... View full answer

Get step-by-step solutions from verified subject matter experts


