Question: show all steps please this is reaction engineering 3. The following reaction is investigated in a constant density batch reactor: The reaction rate is: dtdCA=kCACB2
show all steps please this is reaction engineering
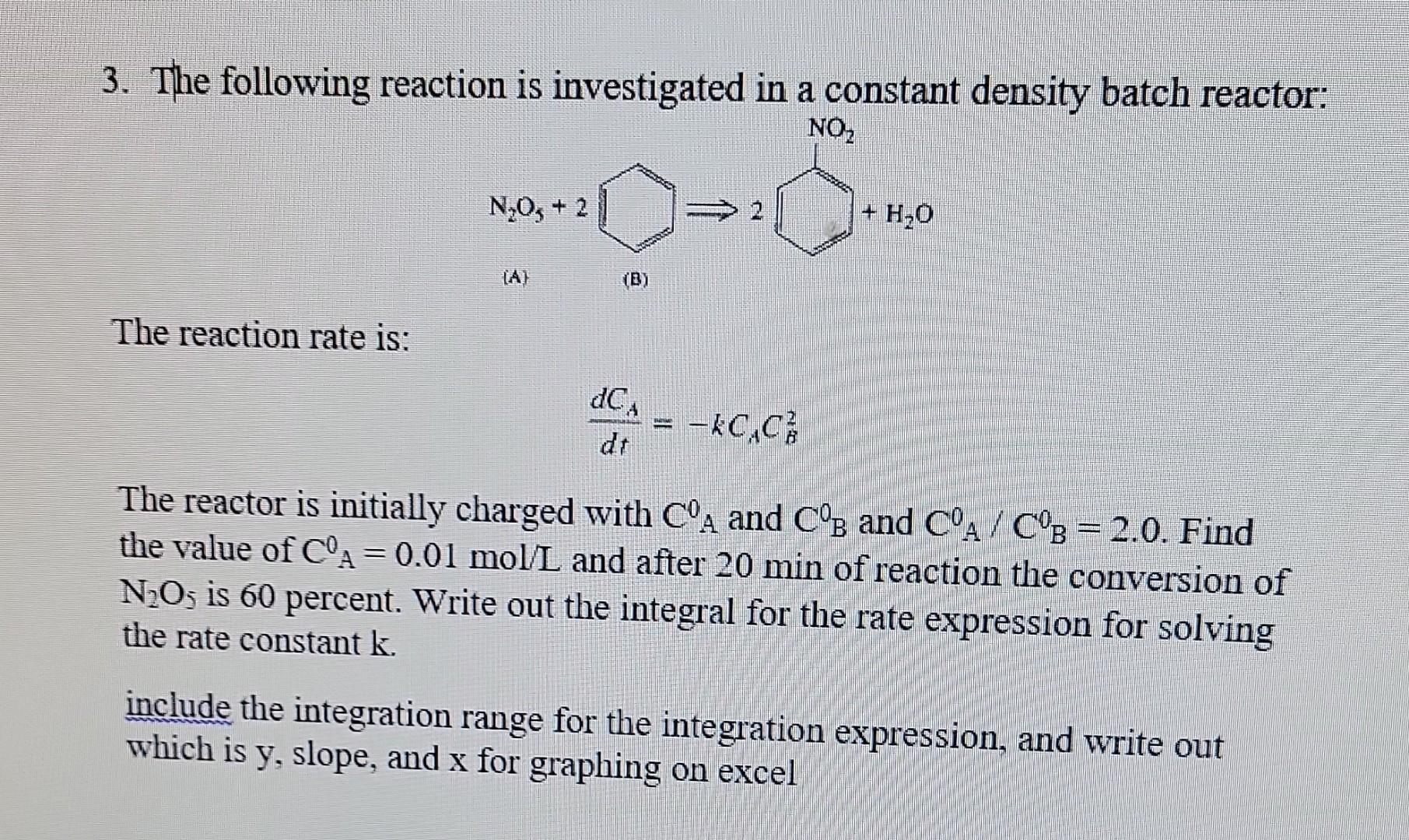
3. The following reaction is investigated in a constant density batch reactor: The reaction rate is: dtdCA=kCACB2 The reactor is initially charged with CA0 and CB and CA0/CB0=2.0. Find the value of CA0=0.01mol/L and after 20min of reaction the conversion of N2O5 is 60 percent. Write out the integral for the rate expression for solving the rate constant k. include the integration range for the integration expression, and write out which is y, slope, and x for graphing on excel
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


