Question: Estimate the entropy change of vaporization of benzene at 323.15 K (50C). The vapor pressure of benzene is given by the equation: 2788.51 In
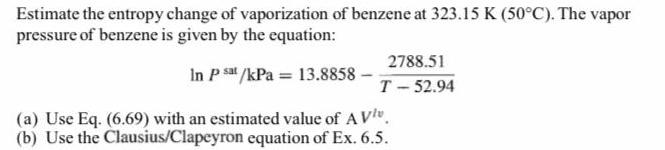
Estimate the entropy change of vaporization of benzene at 323.15 K (50C). The vapor pressure of benzene is given by the equation: 2788.51 In P s /kPa = 13.8858 - T- 52.94 (a) Use Eq. (6.69) with an estimated value of AVl". (b) Use the Clausius/Clapeyron equation of Ex. 6.5.
Step by Step Solution
3.41 Rating (145 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
60d02c45429cd_219584.pdf
180 KBs PDF File
60d02c45429cd_219584.docx
120 KBs Word File


