Question: Since NO2+ is highly reactive, why does it not just react with the water just formed, going back to nitric acid? (There are two correct
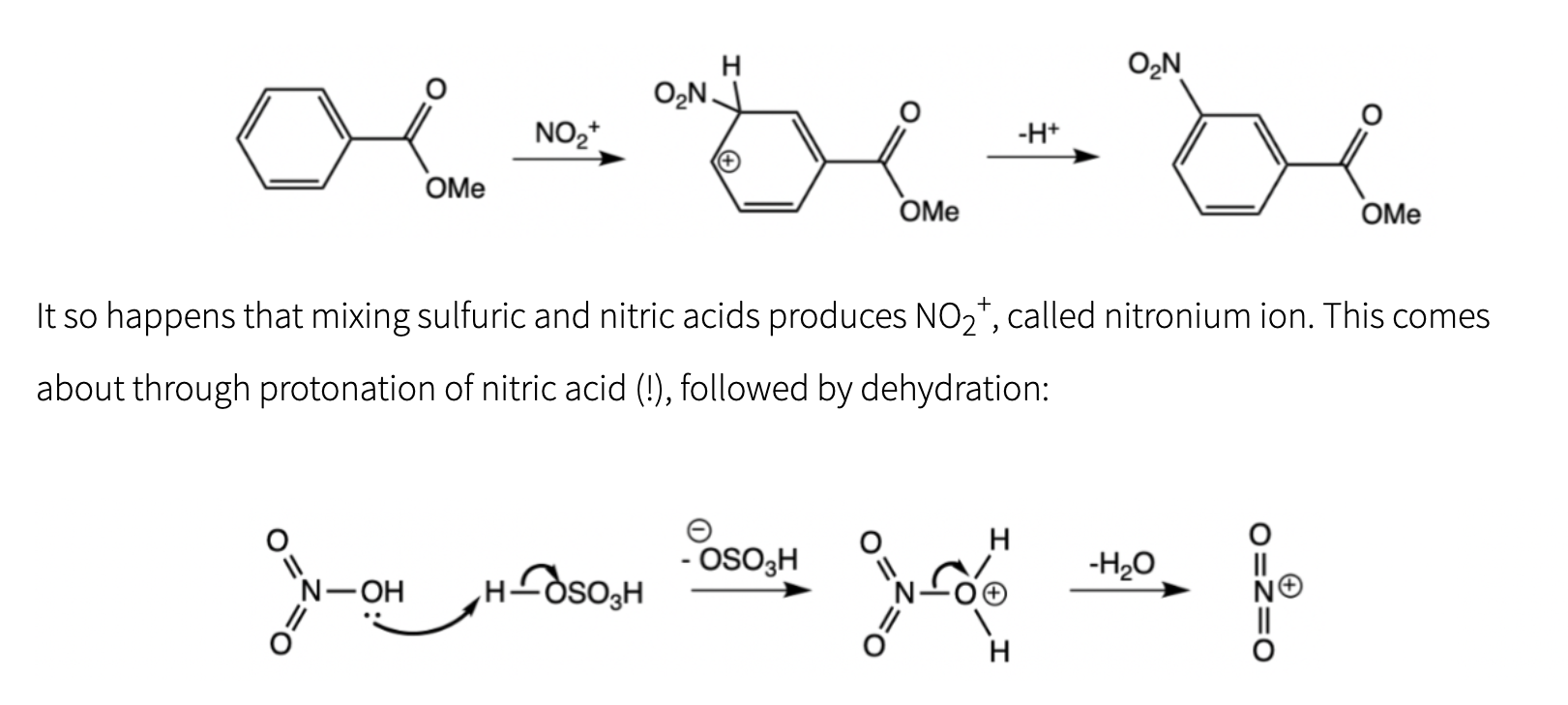 Since NO2+ is highly reactive, why does it not just react with the water just formed, going back to nitric acid? (There are two correct answers)
Since NO2+ is highly reactive, why does it not just react with the water just formed, going back to nitric acid? (There are two correct answers)
- The water is in this case a solute in a strong acid and thus is not available to react.
- Water is a poor nucleophile.
- Steric hindrance prevents this reaction.
- It can react if the water content is above 35%.
(Could you also explain why 2 of these are correct? Thank you!)
H+ It so happens that mixing sulfuric and nitric acids produces NO2+, called nitronium ion. This comes about through protonation of nitric acid (!), followed by dehydration
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


