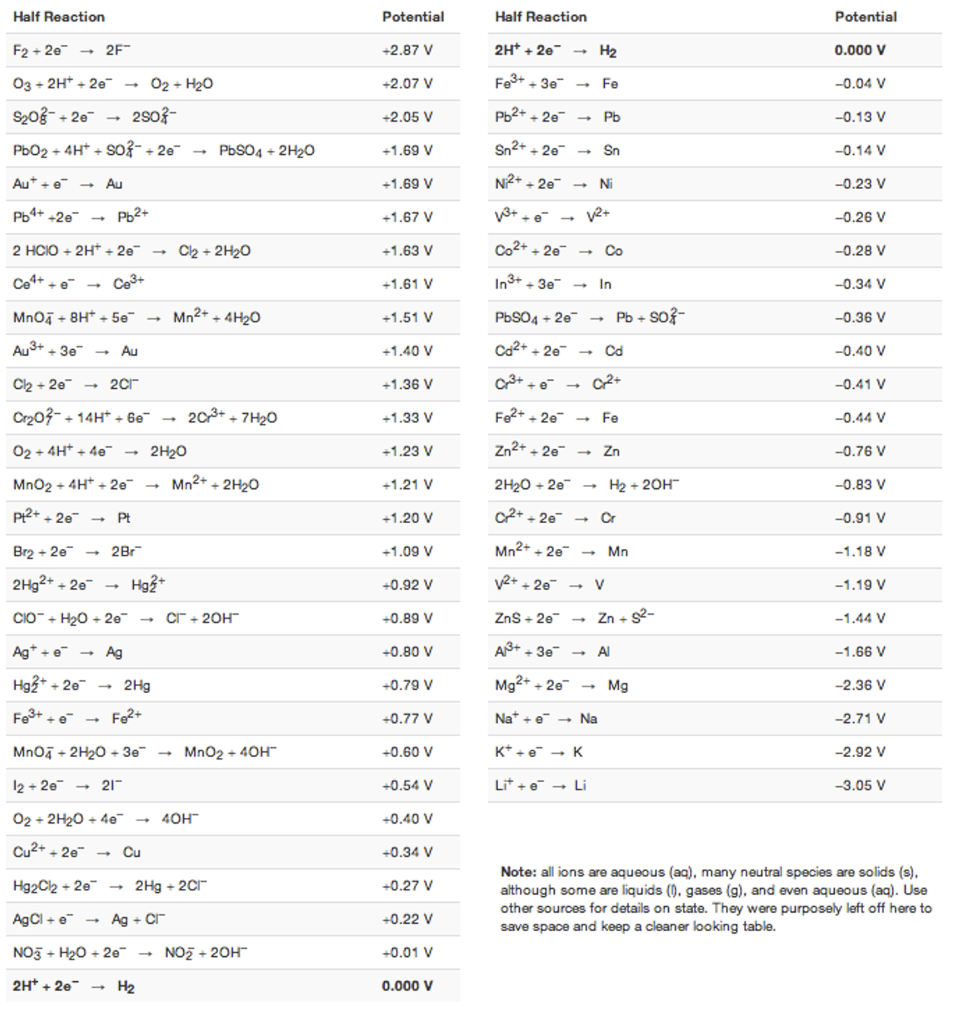
Question: Sn can displace Au + from aqueous solution. Write a balanced redox reaction, and, using the standard potential table attached, calculate the cell potential for
- Sn can displace Au+ from aqueous solution. Write a balanced redox reaction, and, using the standard potential table attached, calculate the cell potential for this process.[6 marks]
Half Reaction Potential Half Reaction Potential F2 +2e-2F +2.87 V 2H+ + 2e - H2 0.000 V +2.07 V Fe3+ + 3e Fe -0.04 V O3 + 2H+ + 2e O2 + H20 S208- + 28- 2003- PbO2 + 4H+ + S03-2e PbSO4 + 2H20 +2.05 V Pb2+ + 2e Pb -0.13 V +1.69 V Sn2+ + 2e Sn -0.14 V Au e- Au +1.69 V -0.23 V N2+ 2e Ni V3+. e- V2+ Pb4+ 2e Pb2+ +1.67 V -0.26 V +1.63 V 8 -0.28 V 2 HCIO + 2H+ + 2e + Cl2 + 2H20 Ce4+ + + Ce 3+ CO2+ + 2e In 3+ + 3e- +1.61 V In -0.34 V MnO2 - BH+ +5e Mn2+ 4H20 - +1.51 V -0.36 V AU3+ + 3e + Au +1.40 V PbSO4 +2e- Pb + S03- Ca2+ + 2e + Cd Cr3+ + 6 (2+ -0.40 V 2cr +1.36 V -0.41 V Cl2 + 20 Cr2O3- + 14H+ +6e" 2013+ + 7H20 -1.33 V Fe2+ 2e- Fe -0.44 V +1.23 V Zn2+ + 2e Zn -0.76 V O2 + 4H+ + 4 + 2H20 MnO2 + 4H+ + 20 Mn2+ + 2H20 P12+ 2e +1.21 V 2H20 +2e H2 + 20" -0.83 V Pt -1.20 V a -0.91 V 02+ + 2e Mn2+ + 20 Br2 +2e 2B +1.09 V - Mn -1.18 V 2Hg2+ + 20 Hg2+ +0.92 V V2+ + 2e V -1.19 V CIO + H20 +2e cr 20H -0.89 V ZnS 2e- Zn +32- -1.44 V 4 -0.80 V A3+ + 3e -1.66 V Ag+ + + Ag Hg2+ + 20 - 2Hg Fe3+ + Fe2+ +0.79 V Mg2+ + 20 Mg -2.36 V +0.77 V Na+ + Na -2.71 V MnO + 2H20 + 3e - MnO2 + 40H -0.60 V K+ eK -2.92 V 12 +2e21 +0.54 V Lite Li -3.05 V O2 + 2H20 + 40 40H +0.40 V Cu2+ + 2e Cu +0.34 V Hg2Cl2 +2e 2Hg 2cr -0.27 V Note: all ions are aqueous (aq), many neutral species are solids (s). although some are liquids (0. gases (g), and even aqueous (aa). Use other sources for details on state. They were purposely left off here to save space and keep a cleaner looking table. AgCl + Ag+cr +0.22 V NO3 + H20 +2e - NO2 + 20" -0.01 V 2H+ + 2 H2 0.000 V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


