Question: Solid calcium carbonate reacts with hydrochloric acid solution according to the following reaction: CaCO3 + 2HCl -----> CaCl2 + CO2 + H2O If 120 mL
Solid calcium carbonate reacts with hydrochloric acid solution according to the following reaction: CaCO3 + 2HCl -----> CaCl2 + CO2 + H2O If 120 mL of hydrochloric acid solution, which is 26.2% by mass and has a density of 1.13 g/mL, is added to a 40.0-g sample of solid calcium carbonate, what will be the molarity of hydrochloric acid in solution when the reaction is complete? (Assume the volume of the solution remains constant.) Atomic masses: Carbon = 12; oxygen = 16; calcium = 40; chlorine = 35.5; hydrogen = 1
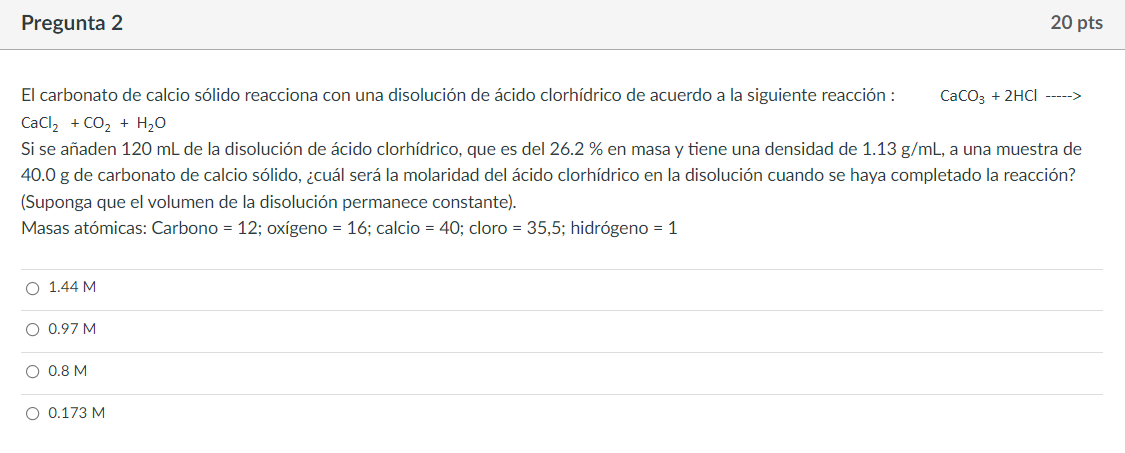
Pregunta 2 20 pts El carbonato de calcio slido reacciona con una disolucin de cido clorhdrico de acuerdo a la siguiente reaccin: CaCO3 + 2HCl -----> CaCl2 + CO2 + H2O Si se aaden 120 mL de la disolucin de cido clorhdrico, que es del 26.2 % en masa y tiene una densidad de 1.13 g/mL, a una muestra de 40.0 g de carbonato de calcio slido, cul ser la molaridad del cido clorhdrico en la disolucin cuando se haya completado la reaccin? (Suponga que el volumen de la disolucin permanece constante). Masas atmicas: Carbono = 12; oxgeno = 16; calcio = 40; cloro = 35,5; hidrgeno = 1 1.44 M O 0.97 M O 0.8 M O 0.173 M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


