Question: Use the References to access important values if needed for this question. The decomposition of hydrogen peroxide in the presence of potassium iodide is believed
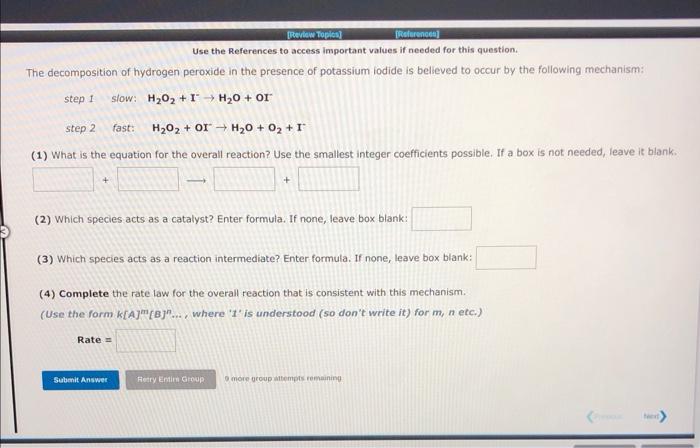
Use the References to access important values if needed for this question. The decomposition of hydrogen peroxide in the presence of potassium iodide is believed to occur by the following mechanism: step I. slow: H2O2+IH2O+OI step 2 fast: H2O2+OIH2O+O2+I (1) What is the equation for the overall reaction? Use the smallest integer coefficients possible. If a box is not needed, leave it blank. (2) Which species acts as a catalyst? Enter formula. If none, leave box blank: (3) Which species acts as a reaction intermediate? Enter formula. If none, leave box blank: (4) Complete the rate law for the overall reaction that is consistent with this mechanism. (Use the form k[A]m[B]n... , where ' t ' is understood (so don't write it) for m, n etc.) Rate =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


