Question: Solve mass transfer Ammonia (A) is diffusing through a stagnant mixture consisting of one-third (1/3) Nitrogen (B) and two- thirds (2/3) Hydrogen (C) by volume.
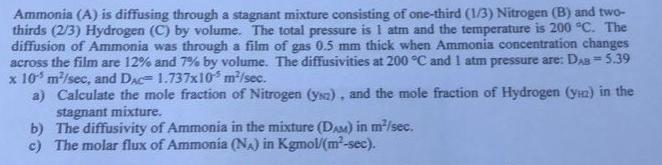
Solve mass transfer
Ammonia (A) is diffusing through a stagnant mixture consisting of one-third (1/3) Nitrogen (B) and two- thirds (2/3) Hydrogen (C) by volume. The total pressure is I am and the temperature is 200 C. The diffusion of Ammonia was through a film of gas 0.5 mm thick when Ammonia concentration changes across the film are 12% and 7% by volume. The diffusivities at 200 C and 1 atm pressure are: Das = 5.39 x 10 m/sec, and Dac= 1.737x10 m/sec. a) Calculate the mole fraction of Nitrogen (yna). and the mole fraction of Hydrogen (ya) in the stagnant mixture. b) The diffusivity of Ammonia in the mixture (DAM) in m/sec. c) The molar flux of Ammonia (NA) in Kgmol/(m-sec)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


