Question: SOLVE THE FOLLOWING PROBLEM USING MATLAB A liquid boils when its vapor pressure equals the external pressure acting on the surface of the liquid. This
SOLVE THE FOLLOWING PROBLEM USING MATLAB
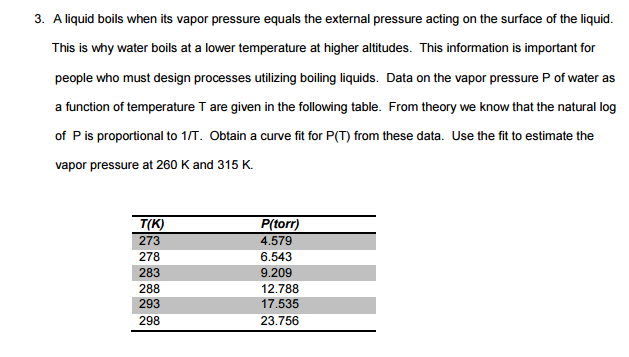
A liquid boils when its vapor pressure equals the external pressure acting on the surface of the liquid. This is why water boils at a lower temperature at higher altitudes. This information is important for people who must design processes utilizing boiling liquids. Data on the vapor pressure P of water as a function of temperature T are given in the following table. From theory we know that the natural log of P is proportional to 1/T. Obtain a curve fit for P (T) from these data. Use the fit to estimate the vapor pressure at 260 K and 315 K. A liquid boils when its vapor pressure equals the external pressure acting on the surface of the liquid. This is why water boils at a lower temperature at higher altitudes. This information is important for people who must design processes utilizing boiling liquids. Data on the vapor pressure P of water as a function of temperature T are given in the following table. From theory we know that the natural log of P is proportional to 1/T. Obtain a curve fit for P (T) from these data. Use the fit to estimate the vapor pressure at 260 K and 315 K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


