Question: solve this by hand P8-6 (4 4h edition) The elementary irreversible organic liquid-phase reaction A+BC is carried out adiabatically in a flow reactor. An equal
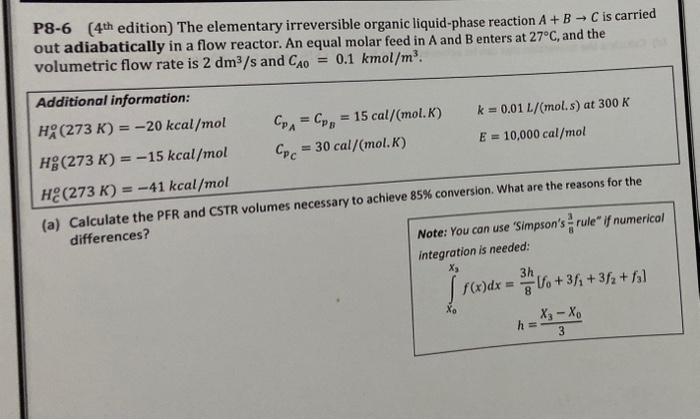

P8-6 (4 4h edition) The elementary irreversible organic liquid-phase reaction A+BC is carried out adiabatically in a flow reactor. An equal molar feed in A and B enters at 27C, and the volumetric flow rate is 2dm3/s and CA0=0.1kmol/m3. Additionalinformation:HA(273K)=20kcal/molHB(273K)=15kcal/molCpA=CpB=15cal/(mol.K)CpC=30cal/(mol.K)k=0.01L/(mol.s)at300KE=10,000cal/mol (a) Calculate the PFR and CSTR volumes necessary to achieve 85% conversion. What are the reasons for the differences? Note: You con use 'Simpson's 83 rule" if numerical integration is needed: x0x3f(x)dx=83h[f0+3f1+3f2+f3]h=3x3x0 (b) What is the maximum inlet temperature one could have so that the boiling point of the liquid (550 K) would not be exceeded even for complete conversion? (d) Calculate the conversion that can be achieved in one 500 -dm 3 CSTR and in two 250-dm 3 CSTRs in series
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


