Question: solve this problem by steps P8-6 (4 th edition) The elementary irreversible organic liquid-phase reaction A+BC is carried out adiabatically in a flow reactor. An
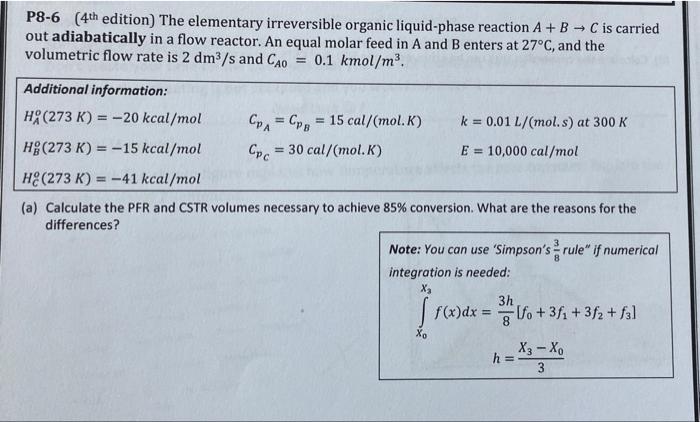
P8-6 (4 th edition) The elementary irreversible organic liquid-phase reaction A+BC is carried out adiabatically in a flow reactor. An equal molar feed in A and B enters at 27C, and the volumetric flow rate is 2dm3/s and CA0=0.1kmol/m3. Additional information: HAo(273K)=20kcal/molHBo(273K)=15kcal/molHCo(273K)=41kcal/molCpA=CpB=15cal/(mol.K)CpC=30cal/(mol.K)k=0.01L/(mol.s)at300KE=10,000cal/mol (a) Calculate the PFR and CSTR volumes necessary to achieve 85% conversion. What are the reasons for the differences? Note: You can use 'Simpson's 83 rule" if numerical integration is needed: X0X3f(x)dx=83h[f0+3f1+3f2+f3]h=3X3X0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


