Question: SOLVE USING EXCEL PLEASE NOT MPATLAB!!!!!! Problem-2 i. Calculate the molar volume of Ammonia by using Van der Waals equation of state (Eq. given below).
SOLVE USING EXCEL PLEASE NOT MPATLAB!!!!!!
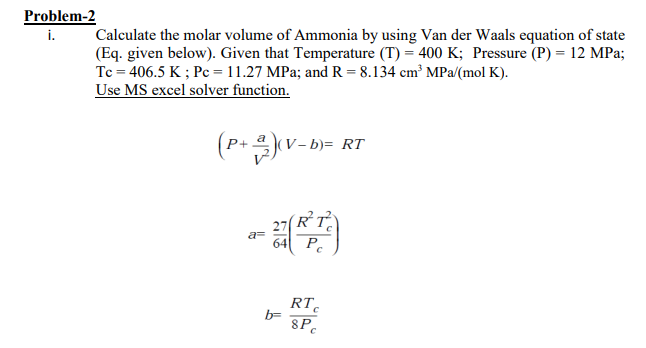
Problem-2 i. Calculate the molar volume of Ammonia by using Van der Waals equation of state (Eq. given below). Given that Temperature (T) = 400 K; Pressure (P) = 12 MPa; Tc = 406.5 K; Pc = 11.27 MPa; and R = 8.134 cm MPa (mol K). Use MS excel solver function. (P+ )(V-b= RT 27 a= 64 RT . b= RTC 8PC
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


