Question: solve using MATLAB Work Problem 5-31 (Gilat) using a for loop to produce the plot, including using different line styles for each plot. The ideal
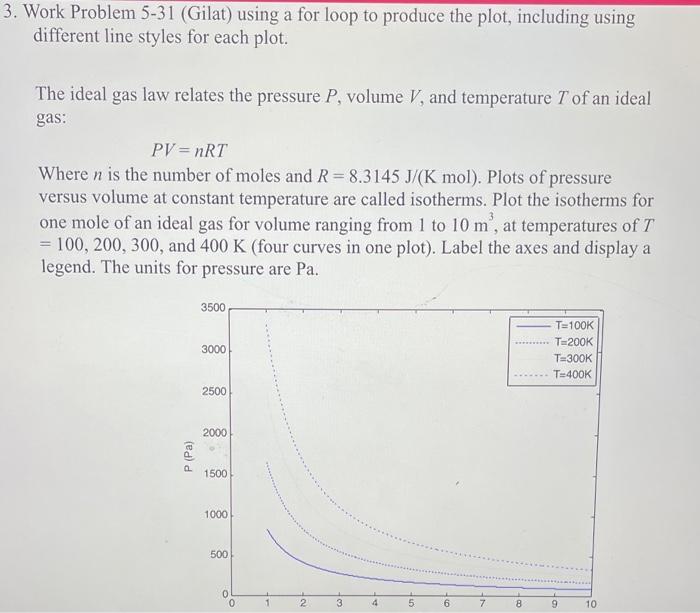
Work Problem 5-31 (Gilat) using a for loop to produce the plot, including using different line styles for each plot. The ideal gas law relates the pressure P, volume V, and temperature T of an ideal gas: PV=nRT Where n is the number of moles and R=8.3145J/(Kmol). Plots of pressure versus volume at constant temperature are called isotherms. Plot the isotherms for one mole of an ideal gas for volume ranging from 1 to 10m3, at temperatures of T =100,200,300, and 400K (four curves in one plot). Label the axes and display a legend. The units for pressure are Pa
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


