Question: Solve using MATLAB, write a clear code please. Chemical reactions often follow the model d c d t = - k c n , where
Solve using MATLAB, write a clear code please.
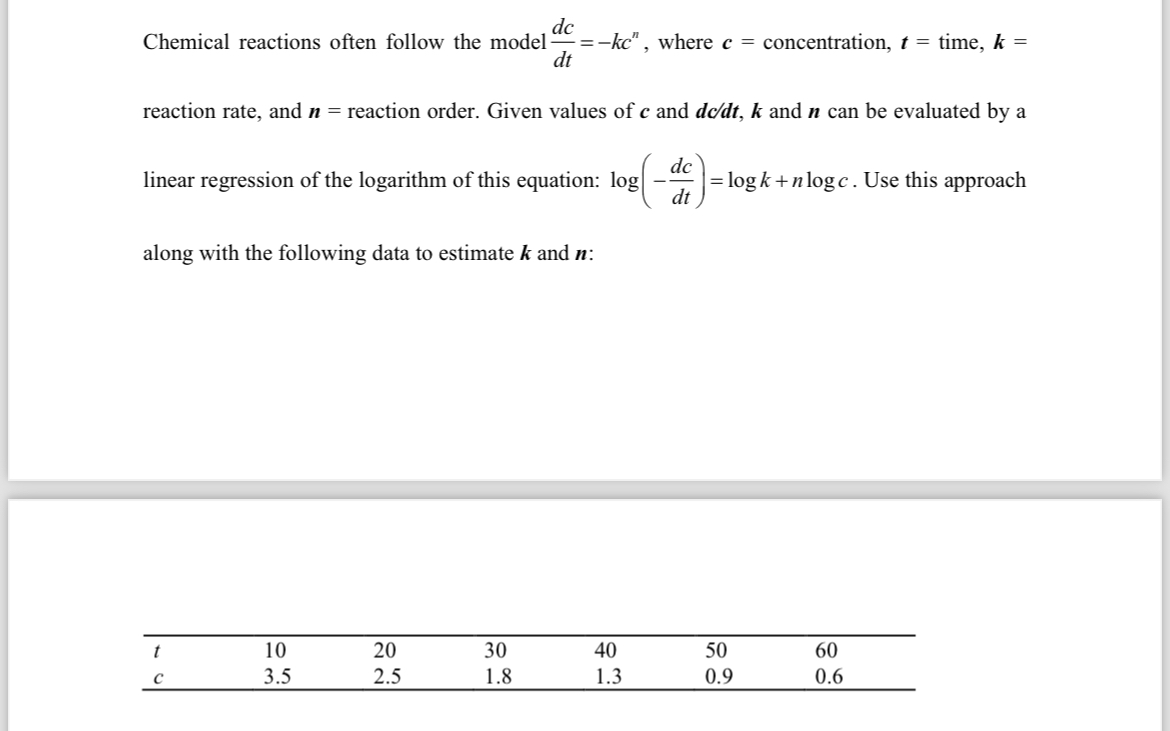
Chemical reactions often follow the model where concentration, time, reaction rate, and reaction order. Given values of and and can be evaluated by a linear regression of the logarithm of this equation: Use this approach along with the following data to estimate and :
table
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


