Question: Step3 a. Draw the curved electron flow arrows for step 2 and step 3 on the scheme above. b. One of the radicals on the
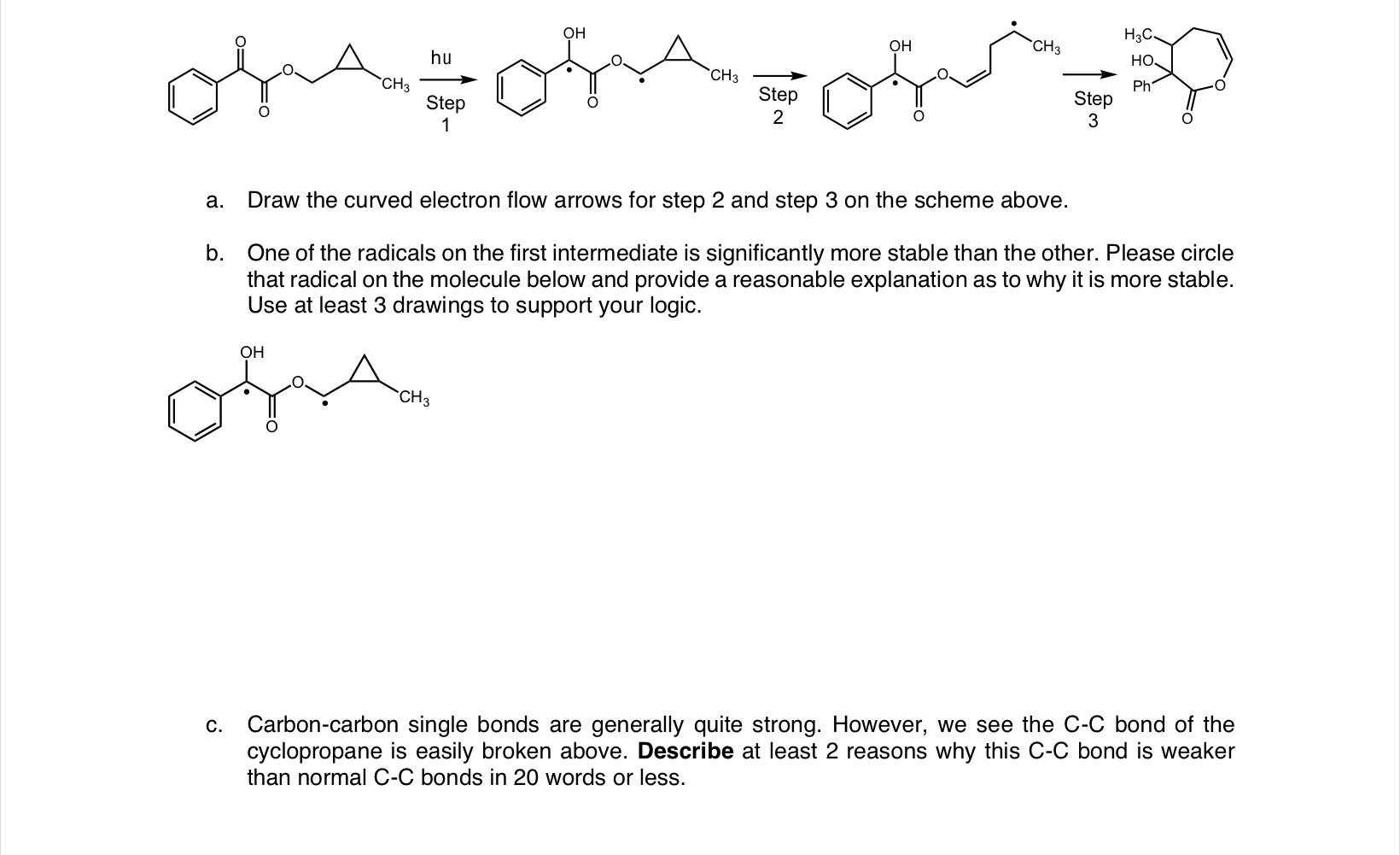
Step3 a. Draw the curved electron flow arrows for step 2 and step 3 on the scheme above. b. One of the radicals on the first intermediate is significantly more stable than the other. Please circle that radical on the molecule below and provide a reasonable explanation as to why it is more stable. Use at least 3 drawings to support your logic. c. Carbon-carbon single bonds are generally quite strong. However, we see the C-C bond of the cyclopropane is easily broken above. Describe at least 2 reasons why this CC bond is weaker than normal C-C bonds in 20 words or less
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


