Question: Suppose also k1k2. That is, the first step is much faster than the second. Write the balanced chemical equation for the overall chemical reaction: Write
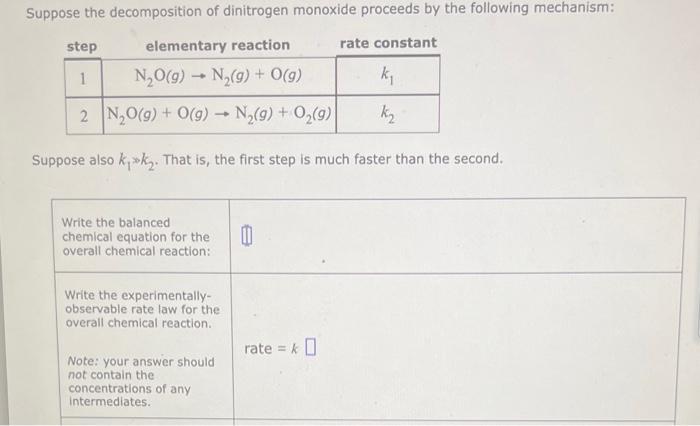

Suppose also k1k2. That is, the first step is much faster than the second. Write the balanced chemical equation for the overall chemical reaction: Write the experimentallyobservable rate law for the overall chemical reaction. Note: your answer should rate =k not contain the concentrations of any intermediates. Express the rate constant k for the overall chemical reaction in terms of k1,k2, and (if necessary) the rate constants k1 and k2 for k= the reverse of the two elementary reactions in the mechanism
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


