Question: Task 2 ( of 2 ) A and B are fed into a reactor to produce C using the chemical formula below. 2 A +
Task of
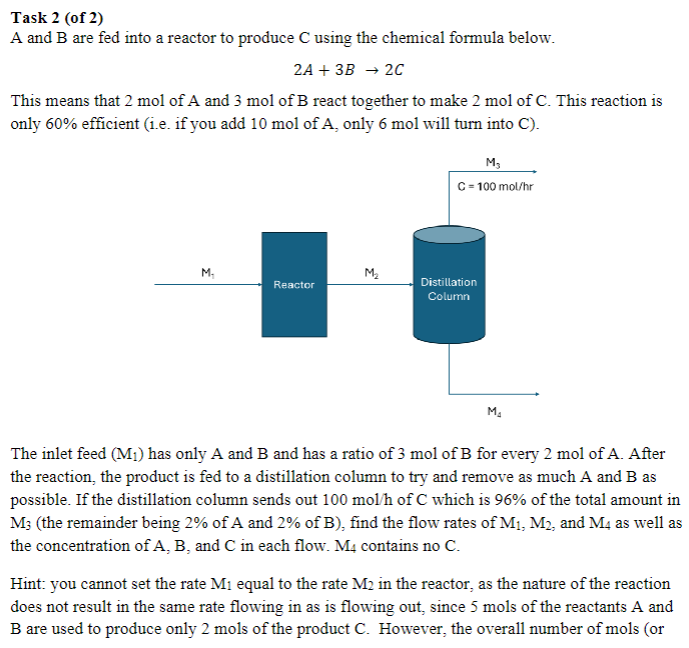
A and are fed into a reactor to produce using the chemical formula below.
This means that mol of A and mol of react together to make mol of This reaction is only efficient ie if you add mol of only mol will turn into C
M
The inlet feed has only A and and has a ratio of mol of for every mol of After the reaction, the product is fed to a distillation column to try and remove as much A and as possible. If the distillation column sends out of which is of the total amount in the remainder being of A and of find the flow rates of and as well as the concentration of and in each flow. contains no
Hint: you cannot set the rate equal to the rate in the reactor, as the nature of the reaction does not result in the same rate flowing in as is flowing out, since mols of the reactants A and are used to produce only mols of the product However, the overall number of mols or
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


