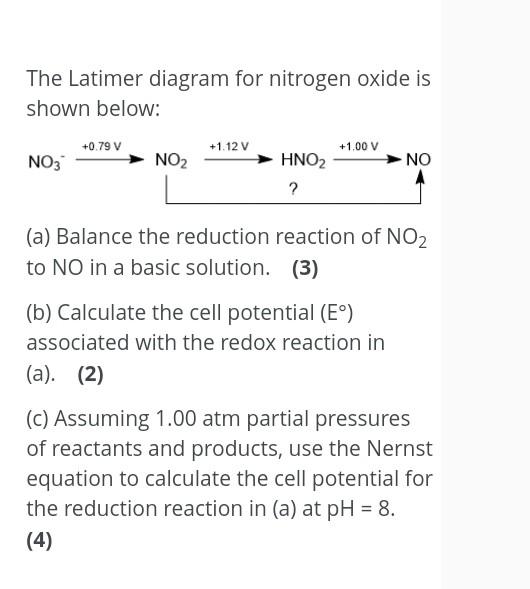
Question: Thank you ... .... .... The Latimer diagram for nitrogen oxide is shown below: +0.79 V +1.12 V +1.00 V NO3 NO2 NO HNO2 ?
Thank you
...
....
....
The Latimer diagram for nitrogen oxide is shown below: +0.79 V +1.12 V +1.00 V NO3 NO2 NO HNO2 ? (a) Balance the reduction reaction of NO2 to NO in a basic solution. (3) (b) Calculate the cell potential (E) associated with the redox reaction in (a). (2) (c) Assuming 1.00 atm partial pressures of reactants and products, use the Nernst equation to calculate the cell potential for the reduction reaction in (a) at pH = 8. (4)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


