Question: The concentration for a zero-order reaction is given by the equation: [A] = -kt + [A] Where: [A] = concentration of reactant A [A], initial
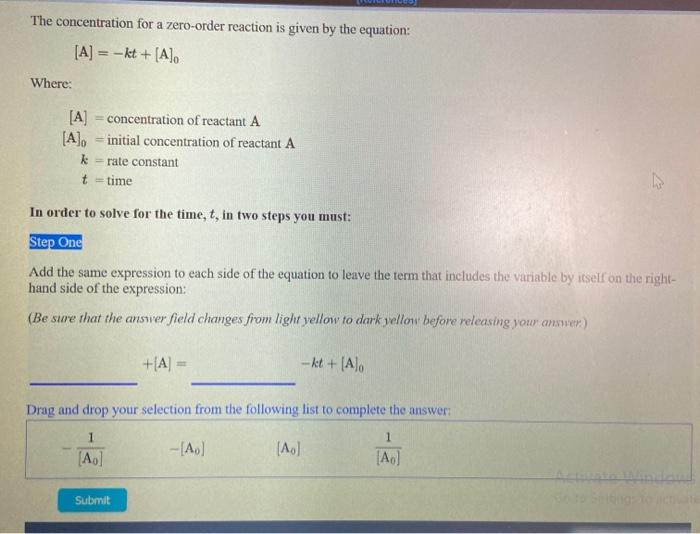
The concentration for a zero-order reaction is given by the equation: [A] = -kt + [A] Where: [A] = concentration of reactant A [A], initial concentration of reactant A k =rate constant t-time In order to solve for the time, t, in two steps you must: Step One Add the same expression to each side of the equation to leave the term that includes the variable by itself on the right- hand side of the expression: (Be sure that the answer field changes from light yellow to dark yellow before releasing your answer) +(A) = - kt + [Alo Drag and drop your selection from the following list to complete the answer 1 1 (Ao] - A) [A] [A] Submit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


