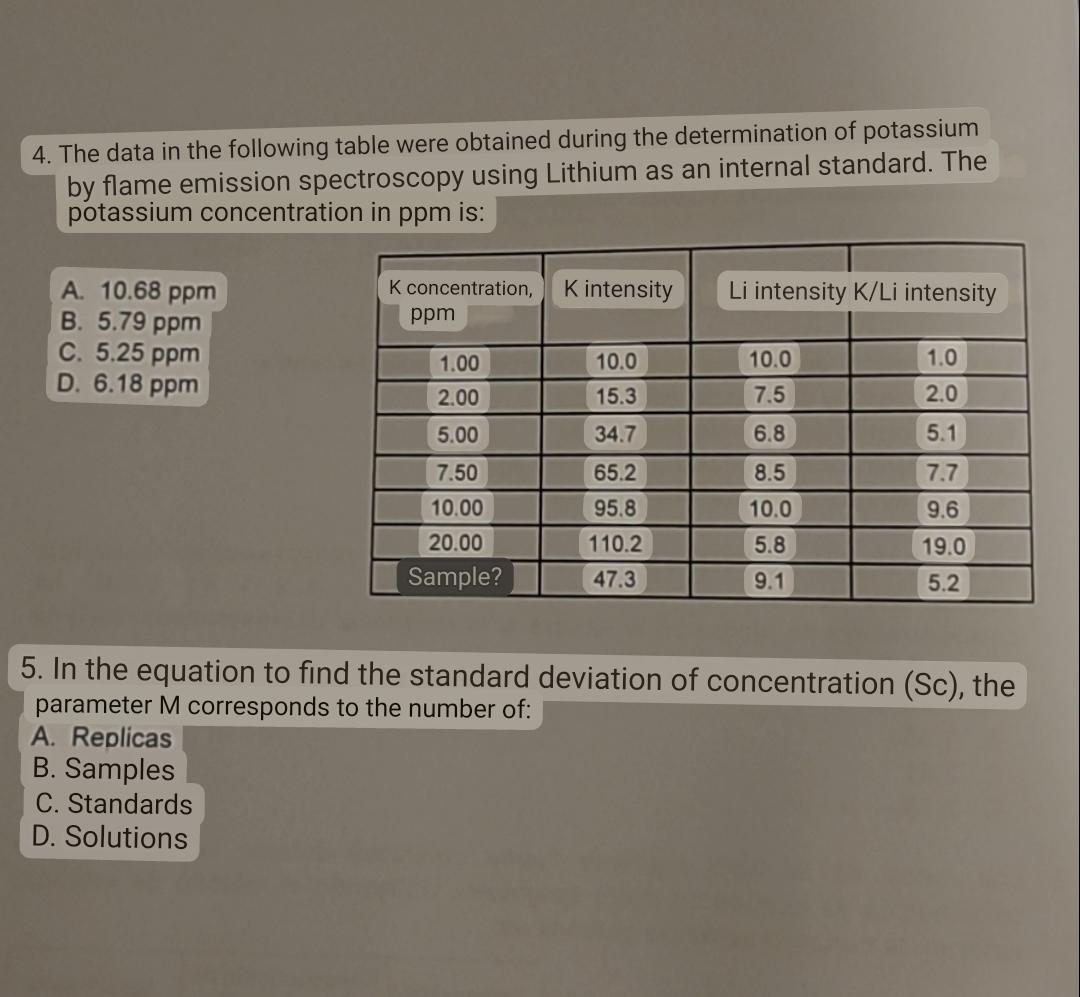
Question: The data in the following table were obtained during the determination of potassium by flame emission spectroscopy using Lithium as an internal standard. The potassium
The data in the following table were obtained during the determination of potassium by flame emission spectroscopy using Lithium as an internal standard. The potassium concentration in ppm is:
A
B
C
D
tabletableK concentration,ppm K intensity,Li intensity KLi intensitySample
In the equation to find the standard deviation of concentration Sc the parameter corresponds to the number of:
A Replicas
B Samples
C Standards
D Solutions
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


