Question: The J=0 to J=1 rotational transition of Carbon monoxide (CO) occurs when it absorbs energy in the microwave region at a frequency (f) 1.153
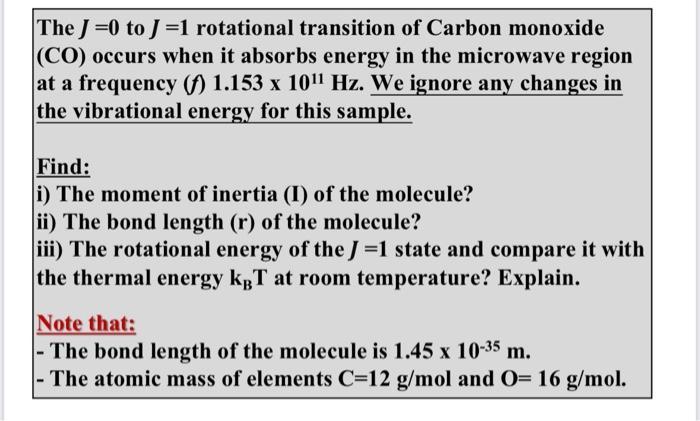
The J=0 to J=1 rotational transition of Carbon monoxide (CO) occurs when it absorbs energy in the microwave region at a frequency (f) 1.153 x 1011 Hz. We ignore any changes in the vibrational energy for this sample. Find: i) The moment of inertia (I) of the molecule? ii) The bond length (r) of the molecule? iii) The rotational energy of the J=1 state and compare it with the thermal energy KBT at room temperature? Explain. Note that: - The bond length of the molecule is 1.45 x 10-35 m. - The atomic mass of elements C=12 g/mol and O= 16 g/mol.
Step by Step Solution
3.49 Rating (159 Votes )
There are 3 Steps involved in it
To tune a PI controller for the given transfer function we need to first determine the process dynamics and then apply the desired tuning method Given ... View full answer

Get step-by-step solutions from verified subject matter experts


