Question: The dynamic response of a reactant concentration in the reactor, CA to a step change in inlet concentration (C'A0) is to be evaluated for an
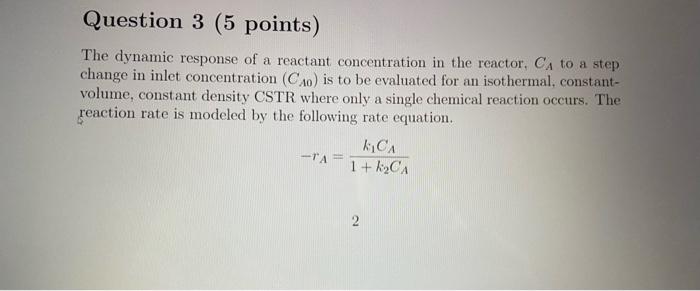
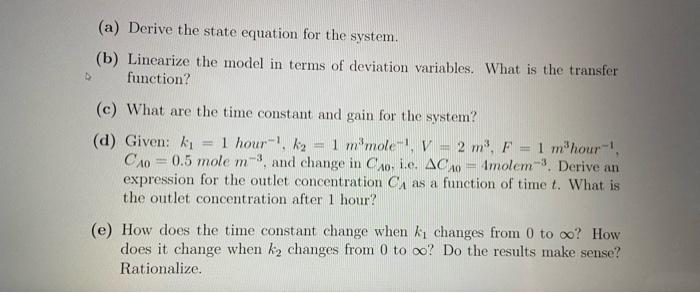
Question 3 (5 points) The dynamic response of a reactant concentration in the reactor, CA to a step change in inlet concentration (CA) is to be evaluated for an isothermal, constant- volume, constant density CSTR where only a single chemical reaction occurs. The reaction rate is modeled by the following rate equation. KICA -TA= 1 + KCA 2 (a) Derive the state equation for the system. (b) Linearize the model in terms of deviation variables. What is the transfer function? D (c) What are the time constant and gain for the system? (d) Given: k 1 hour 1 kg = 1 m mole-I, V = 2 m F = 1 m'hour- CA=0.5 mole m3. and change in Co. i.e. ACA = Imolem-3. Derive an expression for the outlet concentration C as a function of time t. What is the outlet concentration after 1 hour? m 9 (e) How does the time constant change when I changes from 0 to oc? How does it change when k2 changes from 0 to oo? Do the results make sense? Rationalize
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


