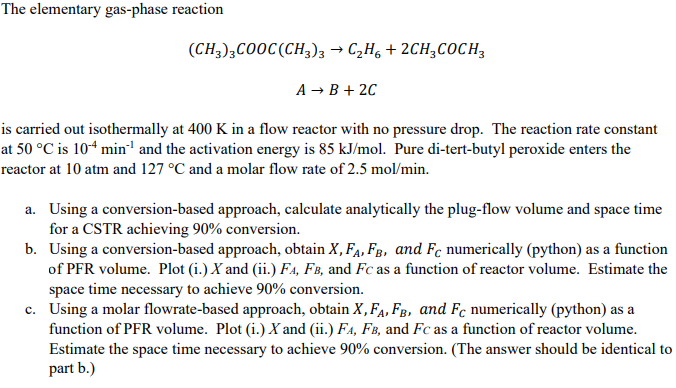
Question: The elementary gas - phase reaction ( C H 3 ) 3 C O O C ( C H 3 ) 3 C 2 H
The elementary gasphase reaction
is carried out isothermally at in a flow reactor with no pressure drop. The reaction rate constant
at is and the activation energy is Pure ditertbutyl peroxide enters the
reactor at atm and and a molar flow rate of
a Using a conversionbased approach, calculate analytically the plugflow volume and space time
for a CSTR achieving conversion.
b Using a conversionbased approach, obtain and numerically python as a function
of PFR volume. Plot i and ii and as a function of reactor volume. Estimate the
space time necessary to achieve conversion.
c Using a molar flowratebased approach, obtain and numerically python as a
function of PFR volume. Plot i and ii and as a function of reactor volume.
Estimate the space time necessary to achieve conversion. The answer should be identical to
part b
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


