Question: The first two spin orbitals for the hydrogen atom are 1 Pr0o(r,0, $,0) = V4n a, ,3/2 and 1. 1(r,0,4,0) e ao V4n a2
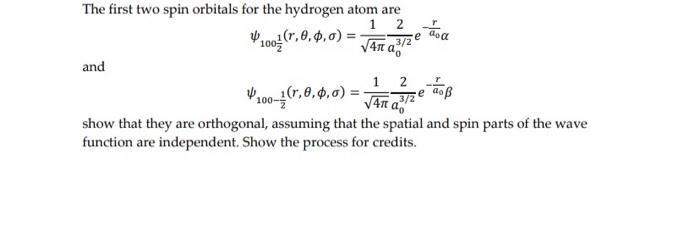
The first two spin orbitals for the hydrogen atom are 1 Pr0o(r,0, $,0) = V4n a, ,3/2 and 1. 1(r,0,4,0) e ao V4n a2 show that they are orthogonal, assuming that the spatial and spin parts of the wave function are independent. Show the process for credits.
Step by Step Solution
3.46 Rating (162 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


